
Chapter 9: Solutions
9.1: Solutions
Solution: A homogeneous mixture in which the solute formly dispersed in a solvent.
Water (H20): It is one of the most common solvents in nature.
It is polar, thus it is a polar solvent.
Solutes and solvents may be solids, liquids, or gases.
The solution that forms has the same physical state as the solvent.
Hydrogen Bonds: Occur between molecules where partially positive hydrogen atoms are attracted to the partially negative atoms.
Hydration: The process of surrounding dissolved ions by water molecules.
9.2: Electrolytes and Non-electrolytes
When electrolytes dissolve in water, the process of dissociation separates them into ions forming solutions that conduct electricity.
When nonelectrolytes dissolve in water, they do not separate into ions and their solutions do not conduct electricity.
Strong electrolyte: There is 100% dissociation of the solute into ions.
Weak Electrolyte: A compound that dissolves in water mostly as molecules.
Equivalent (Eq): The amount of that ion equal to 1 mole of positive or negative electrical charge.
In any solution, the charge of the positive ions is always balanced by the charge of the negative ions.
9.3: Solubility
It is the maximum amount of solute that can be dissolved at a certain temperature.
It is used to describe the amount of a solute that can dissolve in a given amount of solvent
It is usually expressed in grams of solute in 100 g of solvent.
Unsaturated solution: The solution does not contain the maximum amount of solute.
Saturated solution: A solution that contains all the solutes that can dissolve.
Recrystallization: A process that occurs when a solution is saturated, the rate at which the solute dissolves becomes equal to the rate at which the solid forms.
When a saturated solution is carefully cooled, it becomes a supersaturated solution because it contains more solute than the solubility allows.
Henry’s Law: It states that the solubility of a gas in a liquid is directly related to the pressure of that gas above the liquid.
Soluble Salts: Ionic compounds that dissolve in water.
Insoluble Salts: Ionic compounds that do not dissociate into ions in water.
9.4: Concentrations of Solutions
Concentration: The amount of solute dissolved in a certain amount of solution.
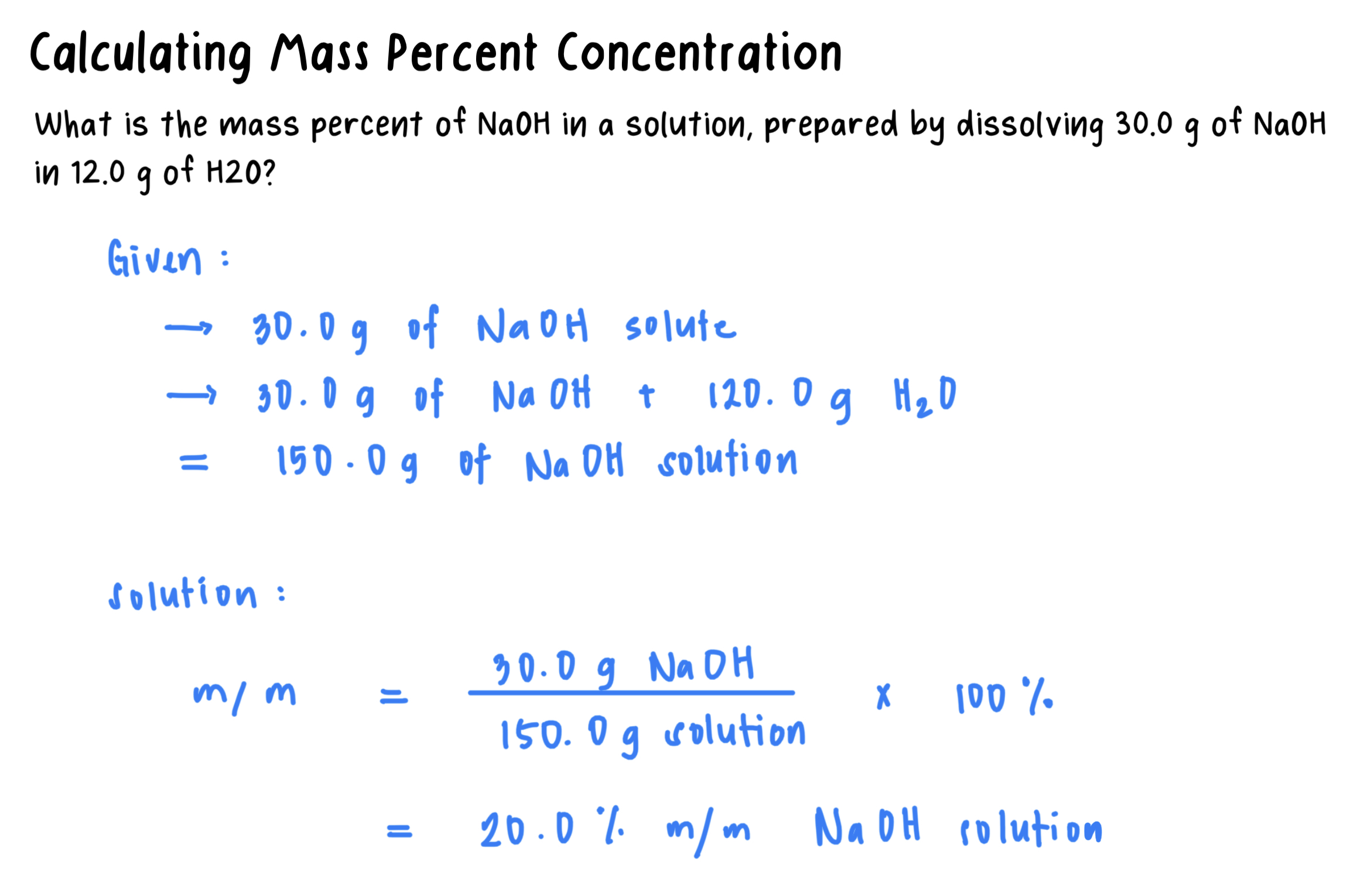
Mass percent (m/m): The mass of the solute in grams for exactly 100 g of solution.
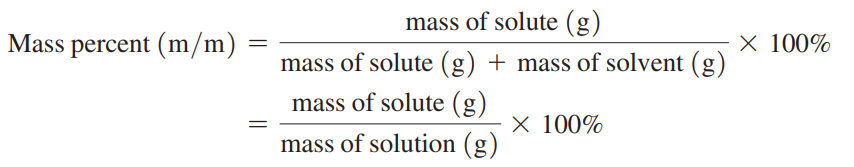
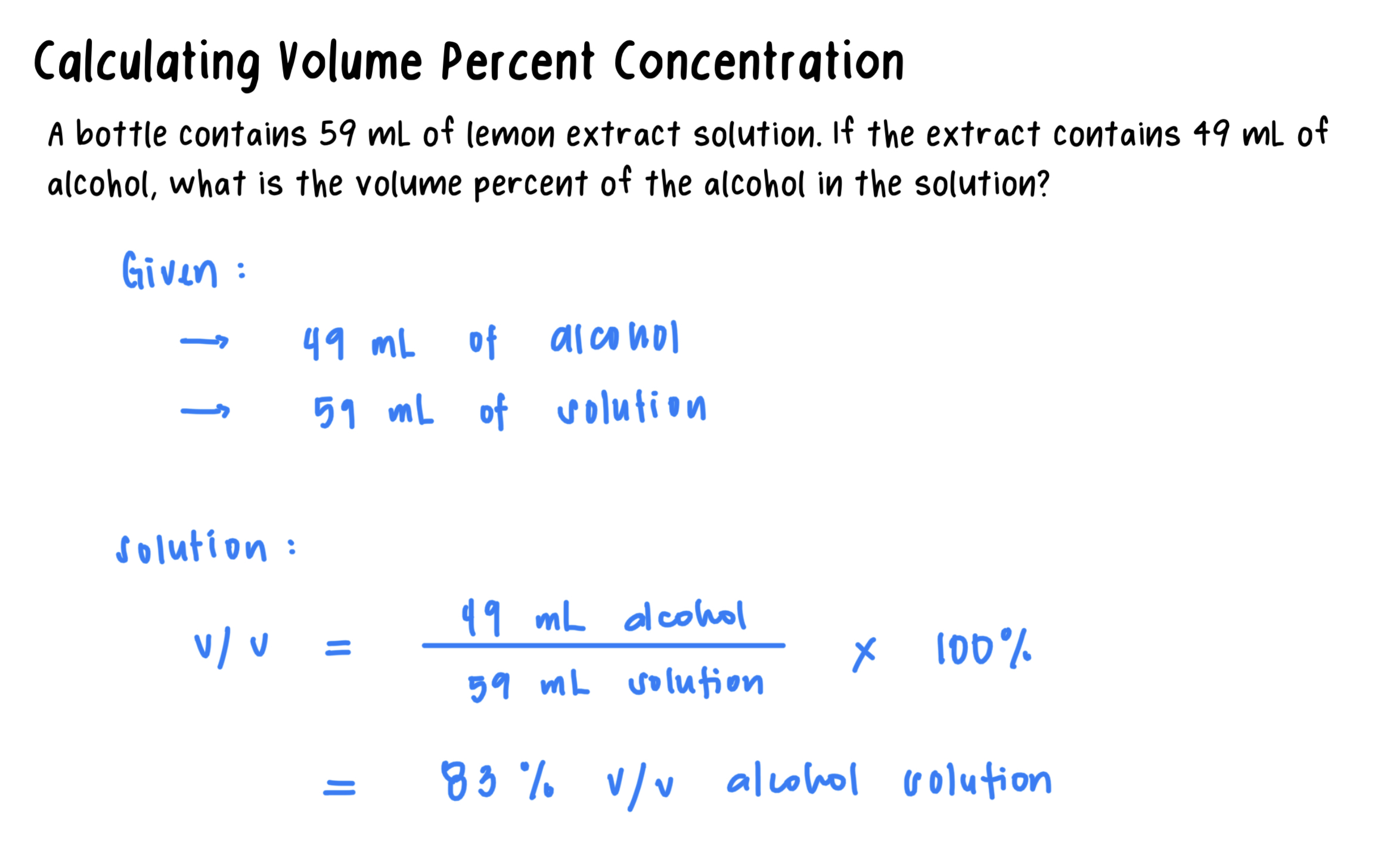
Volume Percent (v/v): The concentration of the volume of liquids or gases.
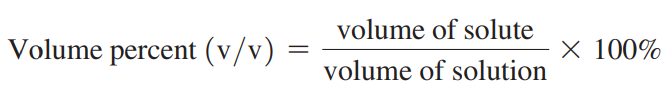
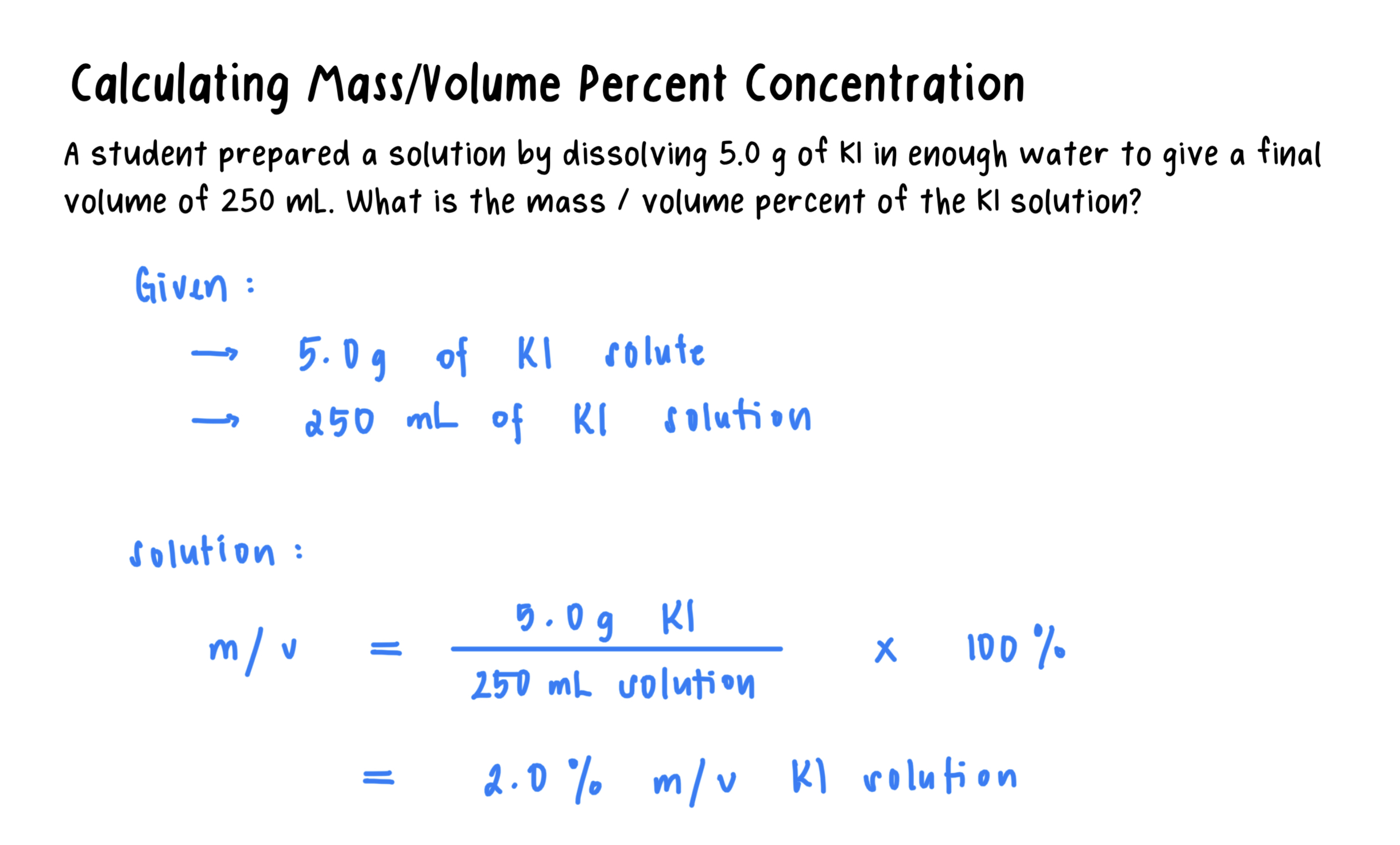
Mass/volume percent: The mass of the solute in grams for exactly 100 mL of solution.
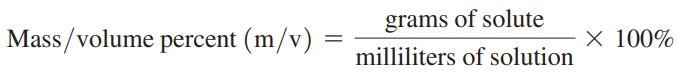
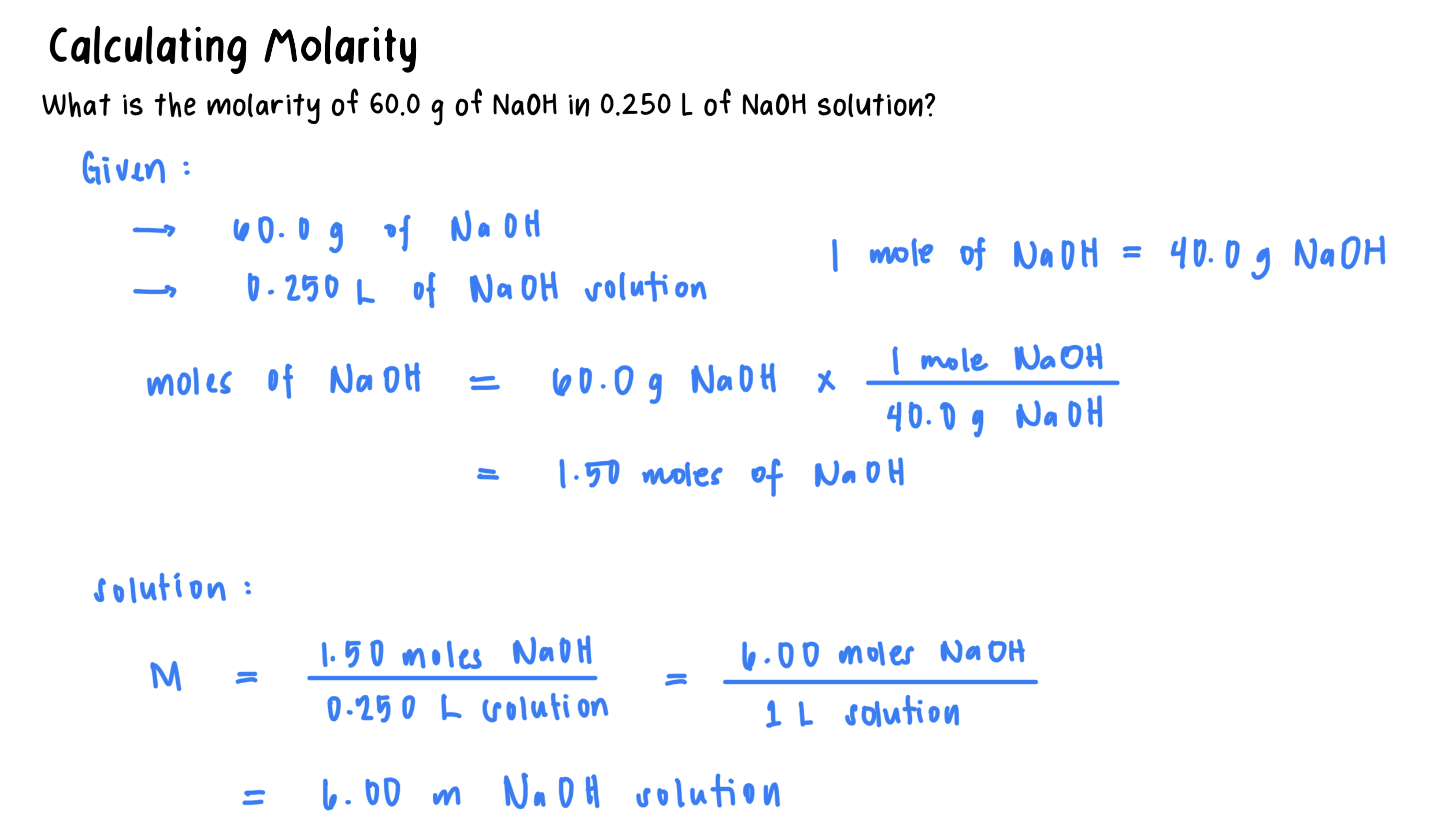
Molarity (M): A concentration that states the number of moles of solute in exactly 1 L of solution.
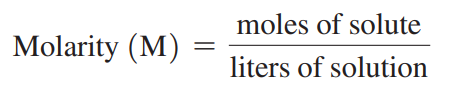
9.5: Properties of Solutions
Dilution: A solvent is added to a solution, which increases the volume.
Solution
It appears transparent, although it may have a color.
The particles are so small that they go through filters and through semipermeable membranes.
A semipermeable membrane allows solvent molecules such as water and very small solute particles to pass through but does allow the passage of large solute molecules.
Colloidal particles
These are large molecules, such as proteins, or groups of molecules or ions.
These are small enough to pass through filters but too large to pass through semipermeable membranes.
Suspension
These are heterogeneous, nonuniform mixtures that are very different from solutions or colloids.
The particles of these are so large that they can often be seen with the naked eye.
Osmosis
The water molecules move through a semipermeable membrane from the solution with the lower concentration of solute into a solution with a higher solute concentration.
Osmotic Pressure: It prevents the flow of additional water into the more concentrated solution.
Reverse Osmosis: A pressure greater than the osmotic pressure is applied to a solution so that it is forced through a purification membrane.
Isotonic Solutions: A solution that has the same particle concentration and osmotic pressure as that of the cells of the body.
Hypotonic Solutions: A solution that has a lower particle concentration and lower osmotic pressure than the cells of the body.
Hemolysis: The increase in fluid causes the cell to swell, and possibly burst.
Hypertonic Solutions: A solution that has a higher particle concentration and higher osmotic pressure than the cells of the body.
Crenation: A process when the water leaves the cell, it shrinks.
Dialysis
A dialyzing membrane, permits small solute molecules and ions as well as solvent water molecules to pass through, but it retains large particles, such as colloids.
It is a way to separate solution particles from colloids.
Chapter 9: Solutions
9.1: Solutions
Solution: A homogeneous mixture in which the solute formly dispersed in a solvent.
Water (H20): It is one of the most common solvents in nature.
It is polar, thus it is a polar solvent.
Solutes and solvents may be solids, liquids, or gases.
The solution that forms has the same physical state as the solvent.
Hydrogen Bonds: Occur between molecules where partially positive hydrogen atoms are attracted to the partially negative atoms.
Hydration: The process of surrounding dissolved ions by water molecules.
9.2: Electrolytes and Non-electrolytes
When electrolytes dissolve in water, the process of dissociation separates them into ions forming solutions that conduct electricity.
When nonelectrolytes dissolve in water, they do not separate into ions and their solutions do not conduct electricity.
Strong electrolyte: There is 100% dissociation of the solute into ions.
Weak Electrolyte: A compound that dissolves in water mostly as molecules.
Equivalent (Eq): The amount of that ion equal to 1 mole of positive or negative electrical charge.
In any solution, the charge of the positive ions is always balanced by the charge of the negative ions.
9.3: Solubility
It is the maximum amount of solute that can be dissolved at a certain temperature.
It is used to describe the amount of a solute that can dissolve in a given amount of solvent
It is usually expressed in grams of solute in 100 g of solvent.
Unsaturated solution: The solution does not contain the maximum amount of solute.
Saturated solution: A solution that contains all the solutes that can dissolve.
Recrystallization: A process that occurs when a solution is saturated, the rate at which the solute dissolves becomes equal to the rate at which the solid forms.
When a saturated solution is carefully cooled, it becomes a supersaturated solution because it contains more solute than the solubility allows.
Henry’s Law: It states that the solubility of a gas in a liquid is directly related to the pressure of that gas above the liquid.
Soluble Salts: Ionic compounds that dissolve in water.
Insoluble Salts: Ionic compounds that do not dissociate into ions in water.
9.4: Concentrations of Solutions
Concentration: The amount of solute dissolved in a certain amount of solution.
Mass percent (m/m): The mass of the solute in grams for exactly 100 g of solution.
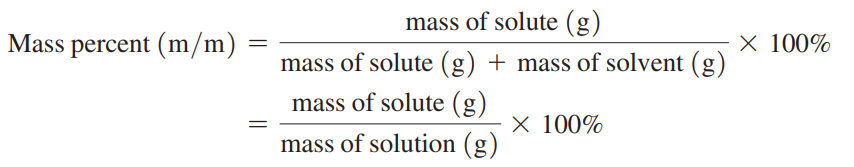

Volume Percent (v/v): The concentration of the volume of liquids or gases.
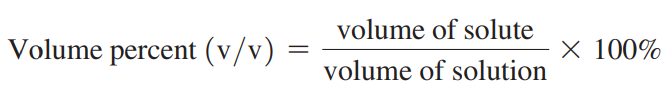

Mass/volume percent: The mass of the solute in grams for exactly 100 mL of solution.
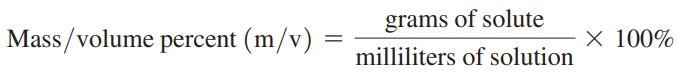

Molarity (M): A concentration that states the number of moles of solute in exactly 1 L of solution.
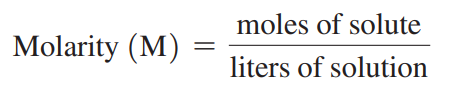

9.5: Properties of Solutions
Dilution: A solvent is added to a solution, which increases the volume.
Solution
It appears transparent, although it may have a color.
The particles are so small that they go through filters and through semipermeable membranes.
A semipermeable membrane allows solvent molecules such as water and very small solute particles to pass through but does allow the passage of large solute molecules.
Colloidal particles
These are large molecules, such as proteins, or groups of molecules or ions.
These are small enough to pass through filters but too large to pass through semipermeable membranes.
Suspension
These are heterogeneous, nonuniform mixtures that are very different from solutions or colloids.
The particles of these are so large that they can often be seen with the naked eye.
Osmosis
The water molecules move through a semipermeable membrane from the solution with the lower concentration of solute into a solution with a higher solute concentration.
Osmotic Pressure: It prevents the flow of additional water into the more concentrated solution.
Reverse Osmosis: A pressure greater than the osmotic pressure is applied to a solution so that it is forced through a purification membrane.
Isotonic Solutions: A solution that has the same particle concentration and osmotic pressure as that of the cells of the body.
Hypotonic Solutions: A solution that has a lower particle concentration and lower osmotic pressure than the cells of the body.
Hemolysis: The increase in fluid causes the cell to swell, and possibly burst.
Hypertonic Solutions: A solution that has a higher particle concentration and higher osmotic pressure than the cells of the body.
Crenation: A process when the water leaves the cell, it shrinks.
Dialysis
A dialyzing membrane, permits small solute molecules and ions as well as solvent water molecules to pass through, but it retains large particles, such as colloids.
It is a way to separate solution particles from colloids.
 Knowt
Knowt
