
Chapter 18: Fires and Explosives
18.1: Fire
Arson – fires that are deliberately set with criminal intent.
The study of fires for forensic purposes involves determining the characteristics and damage caused by the fire as well as the point of origin and cause.
Combustion – the reaction of a fuel with oxygen.
The products of complete combustion are carbon dioxide (and other oxides if present), water, and energy
Exothermic – a chemical reaction that releases energy as one of its products.
Endothermic – a reaction that requires the input of energy for the reaction to take place.
Conditions for a Fire
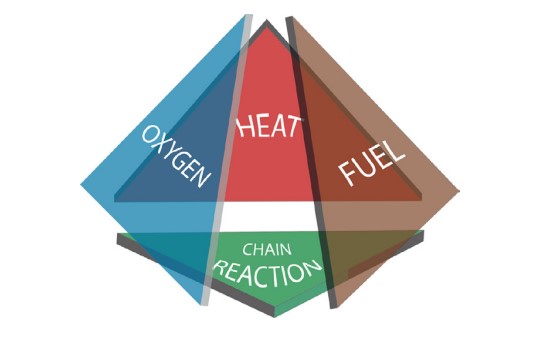
The fire tetrahedron depicts the four elements that must be present to have a fire: a source of heat or energy, fuel, a source of oxygen, and a chain reaction between the fuel and oxygen.
The source of energy is necessary to elevate the fuel and oxygen molecules into an excited state so that they can undergo chemical reactions.
The temperature necessary to do this varies with the fuel and is called the ignition temperature.
Once this temperature is reached, a fire can continue on a self-sustaining basis.
The fuel must be a vapor for it to combust.
Flashpoint – the lowest temperature that will allow a liquid to produce a flammable vapor.
Flame point – the highest temperature.
Flammable and combustible liquids are further subdivided into the following classes:
Class IA
Flashpoint below 73°F (22.8°C) and boiling point below 100 °F (37.8°C).
Acetaldehyde, diethyl ether, pentane, ethyl chloride, ethyl mercaptan, hydrocyanic acid, and gasoline.
Class IB
Flashpoint below 73°F (22.8°C) and the boiling point at or above 100 °F (37.8 °C).
Acetone, benzene, carbon disulfide, cyclohexane, ethyl alcohol, heptane, hexane, isopropyl alcohol, methyl alcohol, methyl ethyl ketone, toluene, petroleum ether, acetonitrile, and tetrahydrofuran.
Class IC
Flashpoint at or above 73 °F (22.8°C) and below 100°F (37.8 °C).
Glacial acetic acid, acetic anhydride, cyclohexanone, and chloroethyl ether.
Class II
Flashpoint at or above 100 °F (37 °C) and below 140 °F (60°C).
Kerosene, diesel fuel, hydrazine, and cyclohexanone.
Class IIIA
Flashpoint at or above 140°F (60°C) and below 200 °F (93.4°C).
Aniline, cyclohexanol, phenol, o-cresol, naphthalene, nitrobenzene, and p-dichlorobenzene.
Class IIIB
Flashpoint at or above 200 °F (93.4 °C).
Diethyl sulfate, diethylene glycol, and p-cresol.
Smoke – occurs when there is incomplete combustion in a fire.
Flashback:
Sometimes a fire will occur in a building where the oxygen supply is limited and as the oxygen is used up, more smoke is formed.
If this fire is then suddenly ventilated, the increased oxygen will cause an explosive fire.
Accelerants – fuels that are easily vaporized and support combustion, and are highly exothermic.
These liquids are poured around the area that is to be burnt and then ignited.
Types of Fire
Natural Fires – are caused by lightning strikes; flammable gases that escape from the ground around an oil field and combust.
Accidental Fires
It’s sometimes hard to distinguish if the cause is either accidental or deliberate.
Sometimes rags that have been used to clean up spills from kerosene or another fuel can heat up enough to cause “spontaneous combustion” if they are left in a closed area for a long time.
Deliberate Fires – started with malicious intent and involve several steps.
Determination of arson can be aided by finding residues from an accelerant or other evidence such as multiple points of origin, fire trails, etc.
Other ways of Classifying Fires
Direct Ignition: It involves the direct application of a spark or flame source to the fuel.
Electrical Fires: These happen due to malfunction of wiring or overheating.
Weather-related Fires: Lightning strikes are the most common. Wildfires are either natural or deliberate.
Mechanical fires: These happen when a machine overheats either through misuse or incorrect placement.
Fire Scene Investigation
The investigator must proceed in an orderly, methodical way, and must make accurate, thorough records of the investigation through still or video photography and good note-taking.
If the fire scene is a building, then the investigation would normally start with the exterior of the building and work toward the point of origin inside the building.
If the fire is outside, such as in a forest, then the investigation proceeds from outside the damaged area toward the area of origin.
Arson dogs – specially trained dogs that can sniff out trace evidence of hydrocarbon accelerants.
Consider the points of entry and exist.
Know the point of origin of the fire.
Start to locate the point of origin.
Low Burning:
Fires generally start in a low area of a building.
Arson fires are seldom started at a high place because the perpetrator may not have a safe point of exit and the damage will generally not be as great since fires burn in an upward direction.
V Patterns: If the point of origin is near a wall or corner of a room, smoke damage on the wall(s) usually occurs in a “V” shape.
Wood charring: The depth of wood charring depends upon the intensity of the heat near the wood and the time of exposure.
Spalling of plaster or concrete: Spalling is the destruction of a surface due to heat or other factors. It usually occurs where the heat is most intense.
Material distortion: Metal and glass may melt or distort owing to high heat.
Soot and smoke staining: The amount of soot present in a fire may indicate the point of origin and the direction of travel of the fire.
Indicate the arson fire.
The presence of an accelerant.
Elimination of natural or accidental causes of a fire.
Fire trails.
Multiple points of origin
Preserve most evidence.
If there is one rule about the packaging fire scene evidence that is to be tested for accelerant residues, it is that airtight containers must be used.
Analyzing Fire Scene Residue Evidence
Isolate the accelerant, which is usually an ignitable liquid or residue from the matrix of charred or unburned material.
Determine the nature of the accelerant residue.
Weathering – used to describe the degradation of an accelerant due to heat or other environmental factors.
Isolation and Concentration of Accelerant Residues
Neat ignitable liquid
It may be possible to pour off the liquid from the residue, filter it to remove solid particulates, separate the hydrocarbon from the water, and make a direct injection into the gas chromatograph.
Partially burned accelerants
The major change that these substances undergo is the evaporation of the most volatile components, leaving the higher boiling components behind. Usually, such exhibits must be extracted from the matrix to be concentrated.
Nearly completely burned accelerants
If an accelerant has been subjected to extreme heat for a significant period, nearly all of the substances present will evaporate or burn. Identification of these residues can be difficult owing to a lack of characteristic chromatographic information.
Four Methods used for Isolation of Accelerant Residues
Headspace Methods – a technique for sampling and examining the volatiles associated with a solid or liquid sample.
Adsorption Methods
Passive Adsorption – a small container of charcoal or Tenax or a plastic strip coated with one of them is placed or suspended inside the container.
Active Adsorption – two tubes containing charcoal or Tenax are inserted partway into the container through holes in the top. Then the air is pumped through one of the tubes into the container.
Solid Phase Microextraction – a fiber made from fused silica is coated with an adsorbent such as charcoal or Tenax. This is inserted into the heated fire residue container.
Solvent Extraction: An evidence container is opened and a small quantity of a suitable solvent is added. The solvent is then poured off and filtered and then evaporated to a small volume leaving behind the accelerant residue
Carbon disulfide is the most popular solvent for this process.
Steam Distillation: Some of the accelerant residues are put in a distillation apparatus with some water, which is then boiled and distilled. The steam will heat and carry over accelerant residues
Analysis of Fire Scene Accelerant Residues by GC
The key to effective analysis of accelerants by GC is to have a comprehensive library of chromatograms that are obtained preferably on the same instrument as the analysis of unknowns, or at least taken under the same conditions.
Mass spectrometry has added flexibility and refinement to GC analysis of fire scene evidence. Individual components of residues can be unequivocally identified.
Selective Ion Monitoring – the mass spectrometer looks for particular ions that are characteristic of particular types of flammables.
Target Compound Analysis – a profile of compounds that are present in each type of accelerant, such as gasoline, are monitored by the mass spectrometer.
In a fire scene investigation, there are two major goals:
Determining the type of fire and
If the fire was deliberately started, who did it?
18.2: Explosions and Explosives
Conflagration – the spark from the spark plug causes very rapid combustion to take place.
ANFO – ammonium nitrate; pellets of it are coated with fuel oil.
It is classified as a low explosive because the velocity of the explosion is not as powerful as in the case of more energetic explosives.
Such materials undergo instantaneous combustion or detonation.
The combustion is not instantaneous, there is always a time lag, but it is even more rapid than in a conflagration.
High explosives such as trinitrotoluene (TNT) and nitroglycerine (NG) undergo detonations.
Effects of Explosions
Blast Pressure: The wave that is created by this blast will shatter anything that gets in its way. The damage decreases with distance as the wave loses energy. As the blast wave travels away from the bomb seat, it creates a partial vacuum because the air itself has been displaced. When the blast wave dissipates, the vacuum must be filled.
Negative Pressure Phase: Not as powerful as the positive pressure blast phase but it is capable of doing additional serious damage to objects that have already been damaged by the initial blast.
Fragmentation Effects
The bomb casing itself can shatter and the pieces can be propelled away from the bomb seat with great force.
The bomber may wrap nails or other pieces of metal around the bomb to create shrapnel that will cause fragmentation damage.
The blast may break up objects in a way that may also fragment and be propelled.
Thermal Effects – least damaging of the effects of the explosion.
At the instant of detonation, a large ball of fire or flash is produced at the bomb seat. This will be very hot and very brief if a high explosive is used and will be longer in duration but not as hot in the case of low explosives.
Types of Explosives
Low Explosives – have detonation velocities below 3280 fps. The main effect of low explosives is to push rather than to shatter.
These kinds of explosives are often used in blasting operations when it is desired to push earth or material out of the way.
The smokeless powder consists of small particles containing nitrocellulose (single base) or nitrocellulose and NG (double base).
Black powder is a finely milled mixture of carbon, sulfur, and potassium nitrate.
Low explosives can be easily set off by using a flame, a spark, or chemicals such as acids.
High Explosives – have detonation rates above 3280fps. These explosives are designed to shatter objects and destroy them.
When these explode they create a strong shock wave that shatters the chemical bonds that hold molecules of fuel and oxygen together.
Two Types of High Explosives:
Initiating high explosives – very powerful and very sensitive. Even the slightest shock or spark can be enough to cause detonation.
Noninitiating high explosives – not sensitive and it usually takes a good deal of effort to cause detonation.
High and Low-Order Explosions
High Order Explosion – one that occurs at or near its maximum theoretical detonation velocity. It is the explosion that you get if everything works out right.
Low Order Explosion – one that takes place at less than optimal efficiency. This can be due any several factors. These include:
Old, out-of-date explosive explosives that is subject to excessive moisture or humidity
Improperly constructed explosive device
Improper placement of the device
Explosive Trains
Low Explosive Trains – One example would be a pipe bomb wherein a fuse made of black powder is used to detonate smokeless powder inside the pipe. This would be classified as a low-explosive train because the final explosive, the smokeless powder, is a low explosive.
High Explosive Trains – The final explosive is usually a secondary explosive. The detonator may be a blasting cap or other suitable primary explosive. In between, there may be other secondary high explosives that act as boosters.
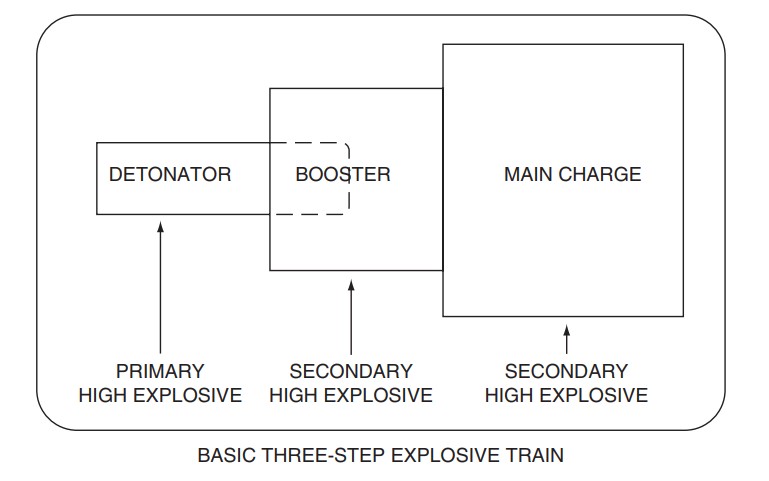
Analysis of Explosives
Vapor Trace Analyzer – a detector that helps aid in sifting through evidence to find explosive residues from the scene.
It is a specialized gas chromatograph that is optimized for explosives.
Electron Capture Detector – used to detect the presence of explosive residues.
Visual Examination – by manually removing residues under a lower power microscope.
If no large particles of undetonated explosive can be isolated, then it may be necessary to dissolve microscopic particles with a suitable solvent and remove them from the debris —- this may be faster than manual sifting but suffers from several disadvantages.
Other methods of analysis
Thin-Layer Chromatography
HPLC
Capillary Electrophoresis
Infrared Spectrophotometry
Chapter 18: Fires and Explosives
18.1: Fire
Arson – fires that are deliberately set with criminal intent.
The study of fires for forensic purposes involves determining the characteristics and damage caused by the fire as well as the point of origin and cause.
Combustion – the reaction of a fuel with oxygen.
The products of complete combustion are carbon dioxide (and other oxides if present), water, and energy
Exothermic – a chemical reaction that releases energy as one of its products.
Endothermic – a reaction that requires the input of energy for the reaction to take place.
Conditions for a Fire

The fire tetrahedron depicts the four elements that must be present to have a fire: a source of heat or energy, fuel, a source of oxygen, and a chain reaction between the fuel and oxygen.
The source of energy is necessary to elevate the fuel and oxygen molecules into an excited state so that they can undergo chemical reactions.
The temperature necessary to do this varies with the fuel and is called the ignition temperature.
Once this temperature is reached, a fire can continue on a self-sustaining basis.
The fuel must be a vapor for it to combust.
Flashpoint – the lowest temperature that will allow a liquid to produce a flammable vapor.
Flame point – the highest temperature.
Flammable and combustible liquids are further subdivided into the following classes:
Class IA
Flashpoint below 73°F (22.8°C) and boiling point below 100 °F (37.8°C).
Acetaldehyde, diethyl ether, pentane, ethyl chloride, ethyl mercaptan, hydrocyanic acid, and gasoline.
Class IB
Flashpoint below 73°F (22.8°C) and the boiling point at or above 100 °F (37.8 °C).
Acetone, benzene, carbon disulfide, cyclohexane, ethyl alcohol, heptane, hexane, isopropyl alcohol, methyl alcohol, methyl ethyl ketone, toluene, petroleum ether, acetonitrile, and tetrahydrofuran.
Class IC
Flashpoint at or above 73 °F (22.8°C) and below 100°F (37.8 °C).
Glacial acetic acid, acetic anhydride, cyclohexanone, and chloroethyl ether.
Class II
Flashpoint at or above 100 °F (37 °C) and below 140 °F (60°C).
Kerosene, diesel fuel, hydrazine, and cyclohexanone.
Class IIIA
Flashpoint at or above 140°F (60°C) and below 200 °F (93.4°C).
Aniline, cyclohexanol, phenol, o-cresol, naphthalene, nitrobenzene, and p-dichlorobenzene.
Class IIIB
Flashpoint at or above 200 °F (93.4 °C).
Diethyl sulfate, diethylene glycol, and p-cresol.
Smoke – occurs when there is incomplete combustion in a fire.
Flashback:
Sometimes a fire will occur in a building where the oxygen supply is limited and as the oxygen is used up, more smoke is formed.
If this fire is then suddenly ventilated, the increased oxygen will cause an explosive fire.
Accelerants – fuels that are easily vaporized and support combustion, and are highly exothermic.
These liquids are poured around the area that is to be burnt and then ignited.
Types of Fire
Natural Fires – are caused by lightning strikes; flammable gases that escape from the ground around an oil field and combust.
Accidental Fires
It’s sometimes hard to distinguish if the cause is either accidental or deliberate.
Sometimes rags that have been used to clean up spills from kerosene or another fuel can heat up enough to cause “spontaneous combustion” if they are left in a closed area for a long time.
Deliberate Fires – started with malicious intent and involve several steps.
Determination of arson can be aided by finding residues from an accelerant or other evidence such as multiple points of origin, fire trails, etc.
Other ways of Classifying Fires
Direct Ignition: It involves the direct application of a spark or flame source to the fuel.
Electrical Fires: These happen due to malfunction of wiring or overheating.
Weather-related Fires: Lightning strikes are the most common. Wildfires are either natural or deliberate.
Mechanical fires: These happen when a machine overheats either through misuse or incorrect placement.
Fire Scene Investigation
The investigator must proceed in an orderly, methodical way, and must make accurate, thorough records of the investigation through still or video photography and good note-taking.
If the fire scene is a building, then the investigation would normally start with the exterior of the building and work toward the point of origin inside the building.
If the fire is outside, such as in a forest, then the investigation proceeds from outside the damaged area toward the area of origin.
Arson dogs – specially trained dogs that can sniff out trace evidence of hydrocarbon accelerants.
Consider the points of entry and exist.
Know the point of origin of the fire.
Start to locate the point of origin.
Low Burning:
Fires generally start in a low area of a building.
Arson fires are seldom started at a high place because the perpetrator may not have a safe point of exit and the damage will generally not be as great since fires burn in an upward direction.
V Patterns: If the point of origin is near a wall or corner of a room, smoke damage on the wall(s) usually occurs in a “V” shape.
Wood charring: The depth of wood charring depends upon the intensity of the heat near the wood and the time of exposure.
Spalling of plaster or concrete: Spalling is the destruction of a surface due to heat or other factors. It usually occurs where the heat is most intense.
Material distortion: Metal and glass may melt or distort owing to high heat.
Soot and smoke staining: The amount of soot present in a fire may indicate the point of origin and the direction of travel of the fire.
Indicate the arson fire.
The presence of an accelerant.
Elimination of natural or accidental causes of a fire.
Fire trails.
Multiple points of origin
Preserve most evidence.
If there is one rule about the packaging fire scene evidence that is to be tested for accelerant residues, it is that airtight containers must be used.
Analyzing Fire Scene Residue Evidence
Isolate the accelerant, which is usually an ignitable liquid or residue from the matrix of charred or unburned material.
Determine the nature of the accelerant residue.
Weathering – used to describe the degradation of an accelerant due to heat or other environmental factors.
Isolation and Concentration of Accelerant Residues
Neat ignitable liquid
It may be possible to pour off the liquid from the residue, filter it to remove solid particulates, separate the hydrocarbon from the water, and make a direct injection into the gas chromatograph.
Partially burned accelerants
The major change that these substances undergo is the evaporation of the most volatile components, leaving the higher boiling components behind. Usually, such exhibits must be extracted from the matrix to be concentrated.
Nearly completely burned accelerants
If an accelerant has been subjected to extreme heat for a significant period, nearly all of the substances present will evaporate or burn. Identification of these residues can be difficult owing to a lack of characteristic chromatographic information.
Four Methods used for Isolation of Accelerant Residues
Headspace Methods – a technique for sampling and examining the volatiles associated with a solid or liquid sample.
Adsorption Methods
Passive Adsorption – a small container of charcoal or Tenax or a plastic strip coated with one of them is placed or suspended inside the container.
Active Adsorption – two tubes containing charcoal or Tenax are inserted partway into the container through holes in the top. Then the air is pumped through one of the tubes into the container.
Solid Phase Microextraction – a fiber made from fused silica is coated with an adsorbent such as charcoal or Tenax. This is inserted into the heated fire residue container.
Solvent Extraction: An evidence container is opened and a small quantity of a suitable solvent is added. The solvent is then poured off and filtered and then evaporated to a small volume leaving behind the accelerant residue
Carbon disulfide is the most popular solvent for this process.
Steam Distillation: Some of the accelerant residues are put in a distillation apparatus with some water, which is then boiled and distilled. The steam will heat and carry over accelerant residues
Analysis of Fire Scene Accelerant Residues by GC
The key to effective analysis of accelerants by GC is to have a comprehensive library of chromatograms that are obtained preferably on the same instrument as the analysis of unknowns, or at least taken under the same conditions.
Mass spectrometry has added flexibility and refinement to GC analysis of fire scene evidence. Individual components of residues can be unequivocally identified.
Selective Ion Monitoring – the mass spectrometer looks for particular ions that are characteristic of particular types of flammables.
Target Compound Analysis – a profile of compounds that are present in each type of accelerant, such as gasoline, are monitored by the mass spectrometer.
In a fire scene investigation, there are two major goals:
Determining the type of fire and
If the fire was deliberately started, who did it?
18.2: Explosions and Explosives
Conflagration – the spark from the spark plug causes very rapid combustion to take place.
ANFO – ammonium nitrate; pellets of it are coated with fuel oil.
It is classified as a low explosive because the velocity of the explosion is not as powerful as in the case of more energetic explosives.
Such materials undergo instantaneous combustion or detonation.
The combustion is not instantaneous, there is always a time lag, but it is even more rapid than in a conflagration.
High explosives such as trinitrotoluene (TNT) and nitroglycerine (NG) undergo detonations.
Effects of Explosions
Blast Pressure: The wave that is created by this blast will shatter anything that gets in its way. The damage decreases with distance as the wave loses energy. As the blast wave travels away from the bomb seat, it creates a partial vacuum because the air itself has been displaced. When the blast wave dissipates, the vacuum must be filled.
Negative Pressure Phase: Not as powerful as the positive pressure blast phase but it is capable of doing additional serious damage to objects that have already been damaged by the initial blast.
Fragmentation Effects
The bomb casing itself can shatter and the pieces can be propelled away from the bomb seat with great force.
The bomber may wrap nails or other pieces of metal around the bomb to create shrapnel that will cause fragmentation damage.
The blast may break up objects in a way that may also fragment and be propelled.
Thermal Effects – least damaging of the effects of the explosion.
At the instant of detonation, a large ball of fire or flash is produced at the bomb seat. This will be very hot and very brief if a high explosive is used and will be longer in duration but not as hot in the case of low explosives.
Types of Explosives
Low Explosives – have detonation velocities below 3280 fps. The main effect of low explosives is to push rather than to shatter.
These kinds of explosives are often used in blasting operations when it is desired to push earth or material out of the way.
The smokeless powder consists of small particles containing nitrocellulose (single base) or nitrocellulose and NG (double base).
Black powder is a finely milled mixture of carbon, sulfur, and potassium nitrate.
Low explosives can be easily set off by using a flame, a spark, or chemicals such as acids.
High Explosives – have detonation rates above 3280fps. These explosives are designed to shatter objects and destroy them.
When these explode they create a strong shock wave that shatters the chemical bonds that hold molecules of fuel and oxygen together.
Two Types of High Explosives:
Initiating high explosives – very powerful and very sensitive. Even the slightest shock or spark can be enough to cause detonation.
Noninitiating high explosives – not sensitive and it usually takes a good deal of effort to cause detonation.
High and Low-Order Explosions
High Order Explosion – one that occurs at or near its maximum theoretical detonation velocity. It is the explosion that you get if everything works out right.
Low Order Explosion – one that takes place at less than optimal efficiency. This can be due any several factors. These include:
Old, out-of-date explosive explosives that is subject to excessive moisture or humidity
Improperly constructed explosive device
Improper placement of the device
Explosive Trains
Low Explosive Trains – One example would be a pipe bomb wherein a fuse made of black powder is used to detonate smokeless powder inside the pipe. This would be classified as a low-explosive train because the final explosive, the smokeless powder, is a low explosive.
High Explosive Trains – The final explosive is usually a secondary explosive. The detonator may be a blasting cap or other suitable primary explosive. In between, there may be other secondary high explosives that act as boosters.

Analysis of Explosives
Vapor Trace Analyzer – a detector that helps aid in sifting through evidence to find explosive residues from the scene.
It is a specialized gas chromatograph that is optimized for explosives.
Electron Capture Detector – used to detect the presence of explosive residues.
Visual Examination – by manually removing residues under a lower power microscope.
If no large particles of undetonated explosive can be isolated, then it may be necessary to dissolve microscopic particles with a suitable solvent and remove them from the debris —- this may be faster than manual sifting but suffers from several disadvantages.
Other methods of analysis
Thin-Layer Chromatography
HPLC
Capillary Electrophoresis
Infrared Spectrophotometry
 Knowt
Knowt
