
Matter & Energy
Matter:
Anything that has mass and takes up space.
Pure Matter
substance
definite
elements/atom type
cannot be broken down
compounds (2+ atoms)
can be broken down
Impure Matter
mixtures
indefinite
Homogenous
uniform
Heterogeneous
not uniform
Models
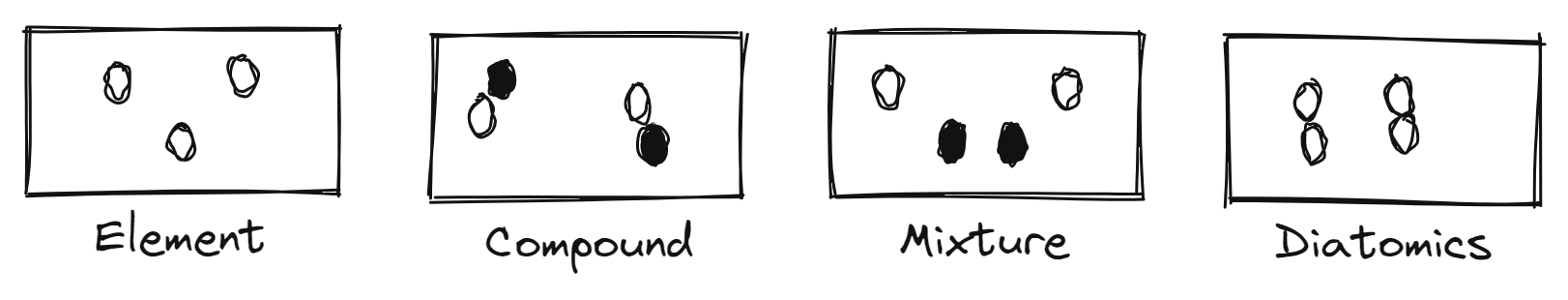 Properties
Properties
Physical
5 senses
mass
solubility
density
volume
odor
phase
Chemical
behavior
flammability
acidity
radioactivity
Separating Mixture
Filtration
heterogenous
different particle size
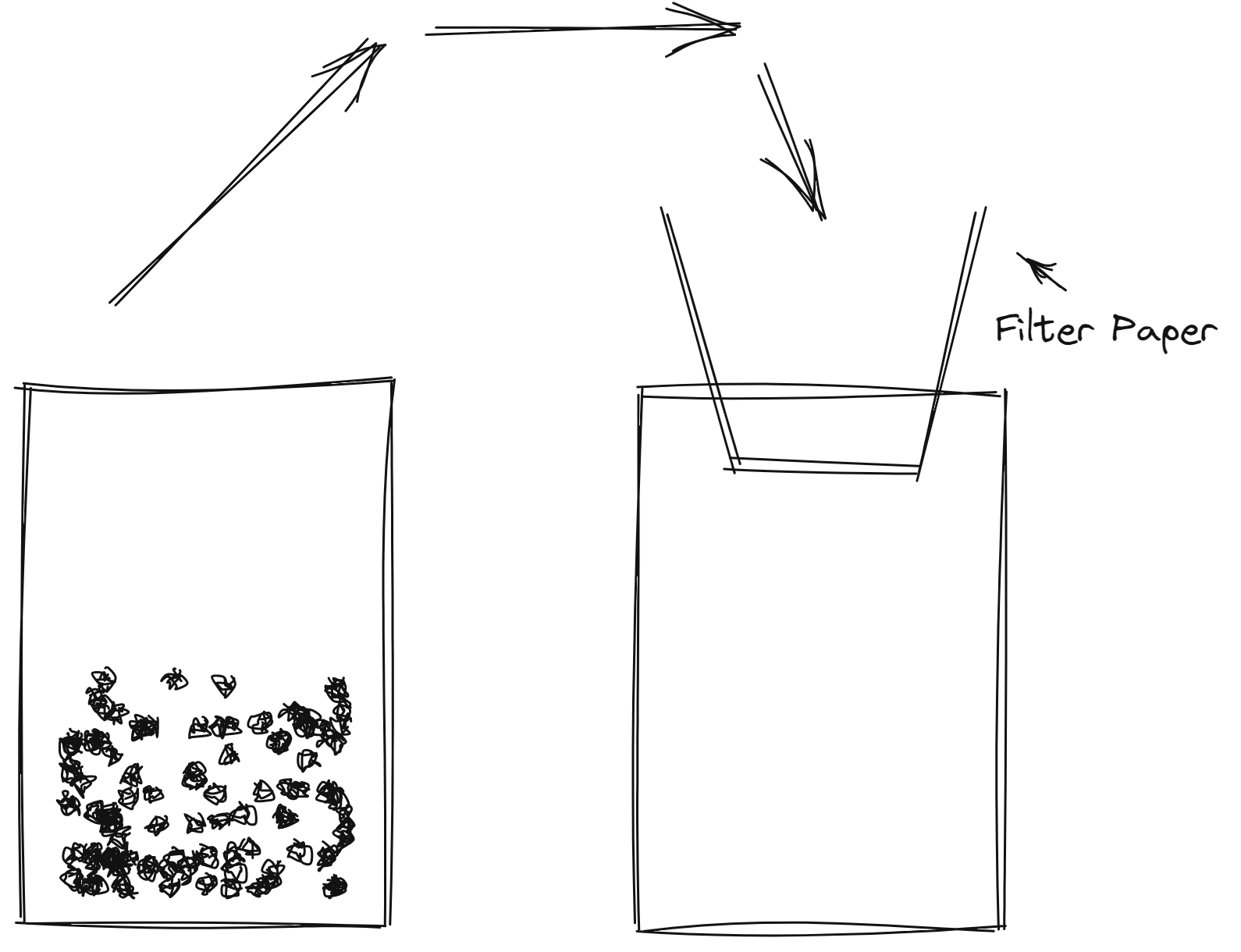
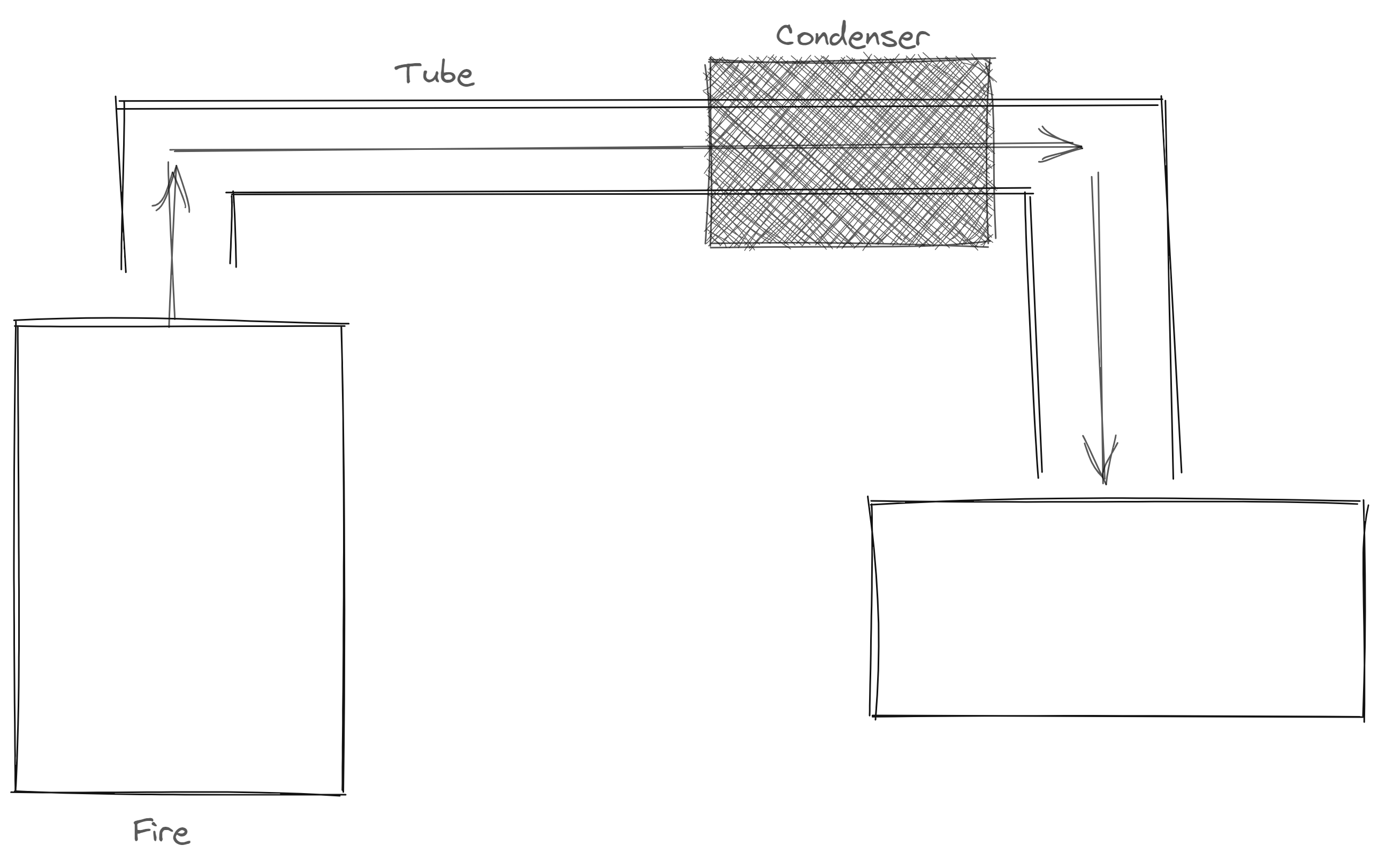
Distillation
homogenous
different boiling point
Chromatography
different molecules in a mixture
solubility

Phases of Matter
Solid
definite volume & shape
crystal & lattice structure
Liquid
definite volume
no definite shape
Gas
chaotic
no definite shape & volume
Energy
ability to do work
The Law of Conservation of Energy: energy cannot be created nor destroyed.
endothermic - absorbed
exothermic - released
Temperature
Q=MCΔT
Q - Heat (Joules)
M - Mass
C - Specific Heat
Water: 4.18 J/g*k
ΔT - Change in Temperature
final temp minus initial temp (Tf - Ti)
High Temperature → More Kinetic Energy → More Movement
Heat travels from High → Low
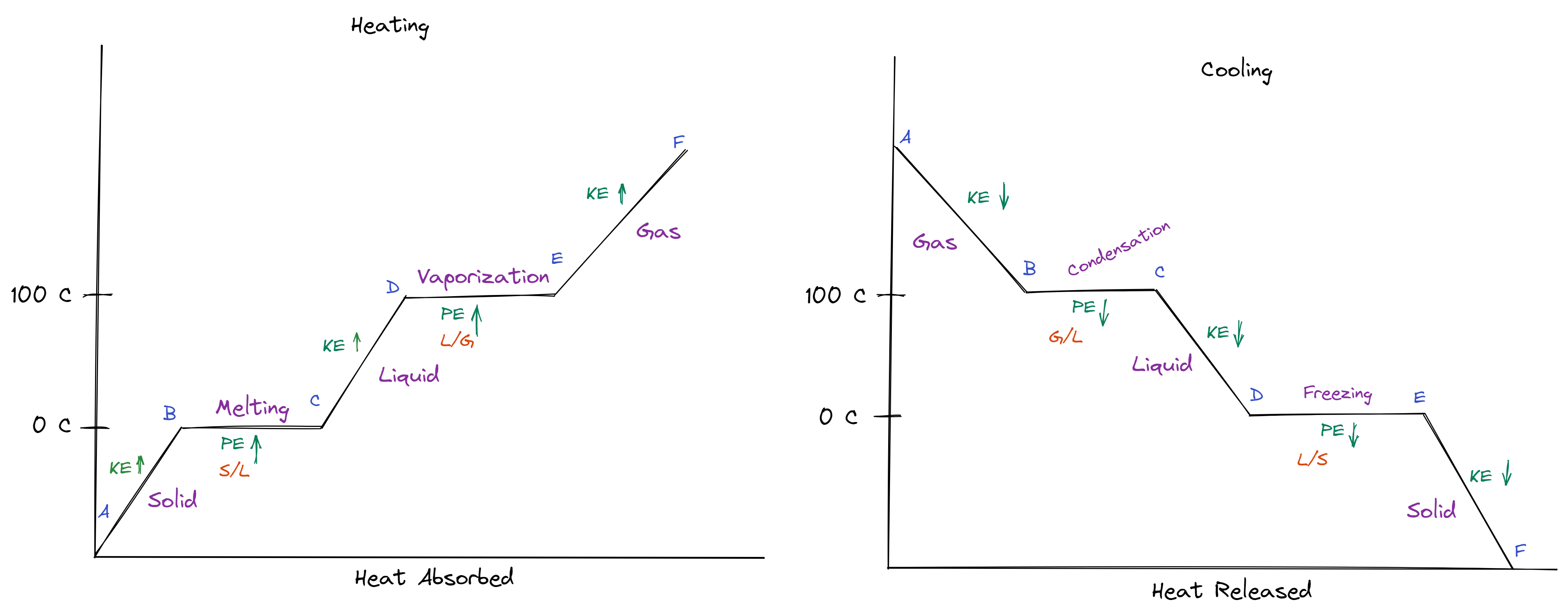
Heating & Cooling Curve
Heating: endothermic
Heat of Fusion: Melting
Q=MhF
hF = 334 J/g
Heat of Vaporization: Vaporization
Q=MhV
hV = 2260 J/g
Cooling: exothermic
Diagrams:
Changes
Physical
change in appearance
does not change in identity
phase changed (solid → liquid → gas)
Chemical
identify
Matter & Energy
Matter:
Anything that has mass and takes up space.
Pure Matter
substance
definite
elements/atom type
cannot be broken down
compounds (2+ atoms)
can be broken down
Impure Matter
mixtures
indefinite
Homogenous
uniform
Heterogeneous
not uniform
Models
 Properties
Properties
Physical
5 senses
mass
solubility
density
volume
odor
phase
Chemical
behavior
flammability
acidity
radioactivity
Separating Mixture
Filtration
heterogenous
different particle size

Distillation
homogenous
different boiling point

Chromatography
different molecules in a mixture
solubility

Phases of Matter
Solid
definite volume & shape
crystal & lattice structure
Liquid
definite volume
no definite shape
Gas
chaotic
no definite shape & volume
Energy
ability to do work
The Law of Conservation of Energy: energy cannot be created nor destroyed.
endothermic - absorbed
exothermic - released
Temperature
Q=MCΔT
Q - Heat (Joules)
M - Mass
C - Specific Heat
Water: 4.18 J/g*k
ΔT - Change in Temperature
final temp minus initial temp (Tf - Ti)
High Temperature → More Kinetic Energy → More Movement
Heat travels from High → Low
Heating & Cooling Curve
Heating: endothermic
Heat of Fusion: Melting
Q=MhF
hF = 334 J/g
Heat of Vaporization: Vaporization
Q=MhV
hV = 2260 J/g
Cooling: exothermic
Diagrams:

Changes
Physical
change in appearance
does not change in identity
phase changed (solid → liquid → gas)
Chemical
identify
 Knowt
Knowt