
1.7 Amines & Amides
Amines
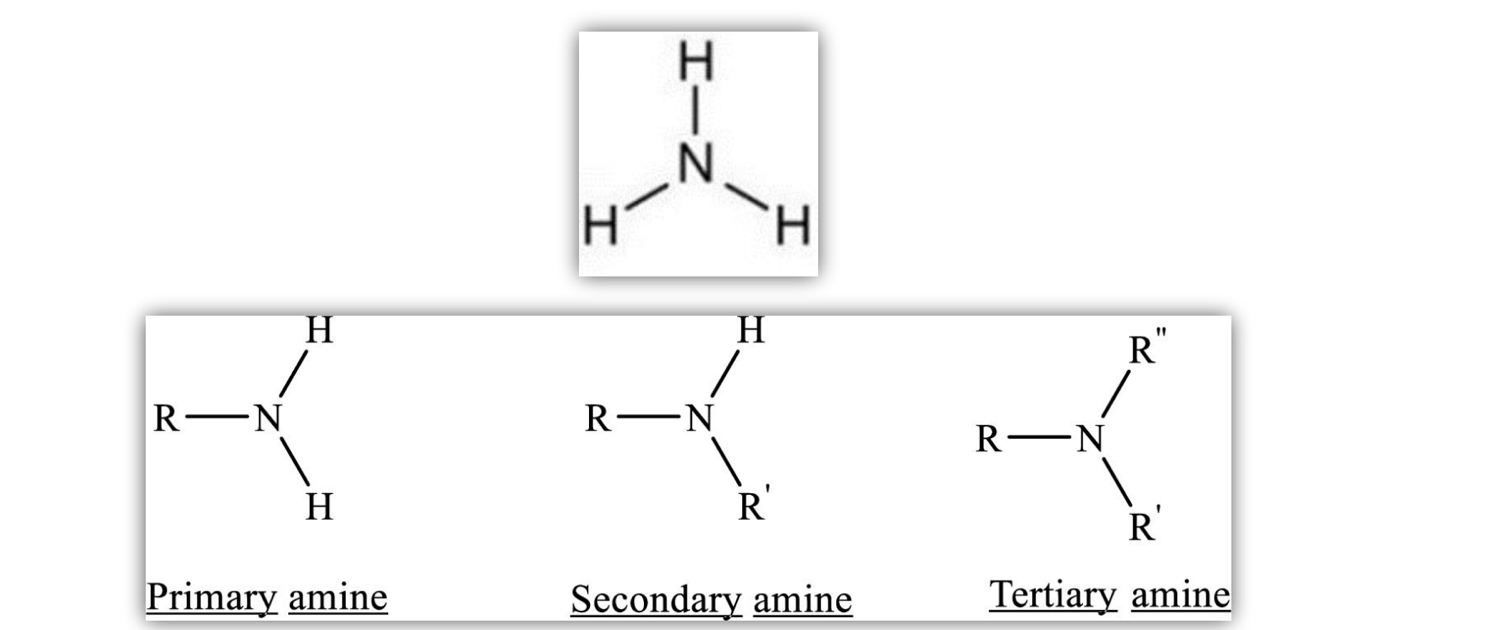
Ammonia (NH3) with alkyl groups replacing hydrogens
Naming Amines
Systemic IUPAC name
alkane name, replace “e” with amine (propanamine)
Common Naming
Use amine as parent chain (propyl amine)
Use amine as branch (aminopropane)
Properties of Amines
Small amines are soluble in water, have higher boiling points than alkanes
N-H bond is polar
Primary amines are most polar, due to the two N-H bonds.
Tertiary amines are least polar, N is surrounded by three non-polar alkyl groups.
Amides
Like esters, but chains are joined by N instead of O
Formed by dehydration reaction between carboxylic acid and amine or ammonia
Naming Amides
Amine becomes alkyl group (N-alkyl)
Acid becomes root, change ending from –oic acid to –amide
1.7 Amines & Amides
Amines
Ammonia (NH3) with alkyl groups replacing hydrogens

Naming Amines
Systemic IUPAC name
alkane name, replace “e” with amine (propanamine)
Common Naming
Use amine as parent chain (propyl amine)
Use amine as branch (aminopropane)
Properties of Amines
Small amines are soluble in water, have higher boiling points than alkanes
N-H bond is polar
Primary amines are most polar, due to the two N-H bonds.
Tertiary amines are least polar, N is surrounded by three non-polar alkyl groups.
Amides
Like esters, but chains are joined by N instead of O
Formed by dehydration reaction between carboxylic acid and amine or ammonia
Naming Amides
Amine becomes alkyl group (N-alkyl)
Acid becomes root, change ending from –oic acid to –amide
 Knowt
Knowt