Unit 10- Organic Chemistry
DO NOT CLICK FLASHCARDS FROM HERE (OR STUDY) Click Here____.
* Tables P, Q, and R on the NYS Chemistry Reference Table are used a lot in this unit. And mentioned a lot in these notes.
*Any code blocks ( ) will represent subscripts. Ex: Carbon Dioxide= CO2
Organic Compounds:
→ They all contain Carbon (C), which is the building block of all life on earth. They are Covalent compounds.
In these compounds the carbons make chains which are also bonded to other elements like Nitrogen(N), Oxygen(O), and Hydrogen(H).
Each carbon can ONLY have 4 bonds. due to having 4 valence electrons, leaving 4 bonding sites.
They can be represented by a Molecular formula, like C
3H8.Or by a structural formula (A Lewis structure)
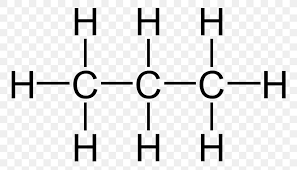
Also a condensed structural formula which writes it out in a line like,
CH
3CH2CH3. Which is the same compound as before (C3H8)This gives some idea as to how a full structural formula for the compound can be drawn.
They can also be written in a stick format where each point of a line represnts a Carbon
The blank spaces would be filled in with hydorgens but arent shown in this notation.
C
3H8would look something like this “/\” becasue the three points are Carbons and the unused bonds would be filled with H.This type of notation most liley wont be used on the NYS Regents.
Characteristics of Organic Compounds:
→ Some characteristics change depending on the compound and its mass/shape. But in general most Organic Compounds share these characteristics in comparison with inorganic compounds (Not containing Carbon)
They are insoluble in water.
most organic compounds are non-polar, and since water is polar it can’t dissolve non-polar compounds. (Like dissolves like)
They do not conduct electricity when dissolved.
They are covalent compounds, and ionic compounds are usually the one which can conduct electricity when dissolved.
They have lower melting points, and boiling points than inorganic compounds
They react a lot slower than inorganic compounds
This is because there is a lot more bond rearranging in Organic compounds compared to Inorganic compounds.
They are also very flammable.
Naming & Functional Groups:
→ When naming organic compounds a lot of the time it comes down to which functional group the compound belongs to. But there are some parts of the name which any organic compounds may share.
Functional Groups → parts of organic molecules which dictate what a certain compound can do
The prefix is linked to the longest un-broken chain of carbons in the compound. These Carbons don’t need to be in a straight line, just an unbroken chain
From there you can use table P on the reference table to find the prefix based on the number.
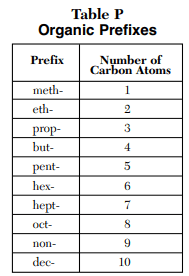
if there is a number followed by a dash before the compound name, it shows where a certain piece of the compound is located. (Will make sense with more examples later on)
“#-name”
From there, the rest of the name depends on the functional group.
Hydrocarbons:
→ This functional group is made of compounds which ONLY contain Carbon and Hydrogen. This is one of the more important groups and has a lot of rules when it comes to naming. And Hydrocarbons Are usually parts of other functional groups.
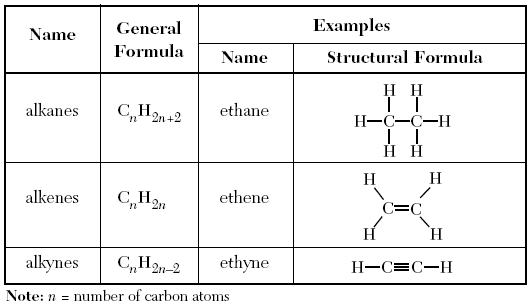
As given on Table Q, the Homologous series of hydrocarbons gives a lot of information on the three types of Hydrocarbons and how they work.
Alkanes → These are Hydrocarbon where there are only single bonds connecting the C’s
to find the number of Hydrogens just plug in the number of C’s in to “n” in the General Formula
H= 2n+2
The name starts with the prefix and ends with “ane” for all alkanes.
#-(pre-fix)ane
They are saturated, which means you can’t add anything else onto the Carbons.
Alkenes → These are Hydrocarbons where at least one C to C bond is a double bond
The equation for the number of Hydrogens in this compound is just: H= 2n
For compounds with three or more C’s, and number followed by a dash is used to show where the double bond is.
It is also sometimes not written if the double bond is on the first carbon.
If it is though the number out front would just be 1.
For example the compound name “3-pentene” means that on the third carbon there is a double bond. Or think of it as the the third bond is a double bond.
You could the Carbons from left to right OR right to left either way is correct.
all Alkenes end in “ene”
#-(pre-fix)ene
Alkynes → Hydrocarbons where one of the C to C bonds is a triple bond.
To find the number Hydrogen the equation is: H=2n-2
The number and dash out front is the same with Alkenes, but instead reprenstes the location of the triple bond.
All Alkynes end in “yne”
#-(pre-fix)yne
Both Alkenes, and Alkynes are unsaturated, meaning you could add more elements onto them.
So to name hydrocarbons you must know which type it is.
Examples:
A hydrocarbon with the longest carbon chain being 4, and having a double bond attached to the second carbon would be named: (How many hydrogens? with only 4 carbons)
2-butene
H= 2n= 2(4)= 8
A hydrocarbon which only has single bond and whose longest carbon chain is 8 would be named:(How many hydrogens? with only 8 carbons)
octane
H= 2n+2 = 2(8)+2= 18
A hydrocarbon with a triple bond on the 4th Carbon, with the longest chain of Carbons being 9 would be named: (How many hydrogens? with only 9 carbons)
4-nonyne
H= 2n-2=2(9)-2= 16
Methyl, Ethyl, and Propyl groups:
→ These are not functional groups, but they are Hydrocarbon groups which can attach to a Carbon in an organic compound. You must memorize these.
Methyl groups → A CH
3tacked onto a carbonMeth-, is the prefix for "one” b/c there is only one extra carbon for this group.
In the name of a compound you put a number followed by a dash behind the name ethyl, and then you write the name of the rest the compound after that.
The number represents the carbon where the ethyl group is attached.
An example would be 2-methyl butane.
If there are 2 ethyl groups in the same compound you use the word diethyl
then you write two numbers in front to show which to carbons the ethyls are attached to.
An example would be 3,4- methyl nonane, or if they are on the same carbon 3,3-methyl nonane.
Ethyl groups → A CH
2CH3/ C2H5attached onto a carbon.Eth-, means 2, so 2 carbons are attached.
Same rules for naming just with the word Ethyl instead of methyl.
Examples are: 2-ethyl pentane, or 2, 3-ethyl hexane, or 3, 3- ethyl nonane
Propyl Groups → A CH
2CH2CH3/ C3H7attachedprop-, means 3, so 3 extra carbons
Again same rules for naming.
If one of these is attached to a alkene or alkyne, you must add the number for where the double or triple bond is ASWELL AS the number of the methyl, ethyl, or propyl group location.
For Example: 2-methyl 3-hexene
Isomers:
→ Compounds with the same molecular formula, but different structures/ shapes.
Often these are made with the use of methyl, ethyl, and propyl groups.
For example an isomer of pentane would be 2-methyl butane
They both have a the same molecular formula (C5H12), but different structural formulas)
2-methyl butane could also be called isopentane in this case
Most compounds have multiple isomers, so just giving them their name with methyl, ethyl, or propyl is easier than keeping track of prefixes.
Other Functional Groups:
→ On table R there is a list of functional groups which the regents could ask about, and how they generally look.
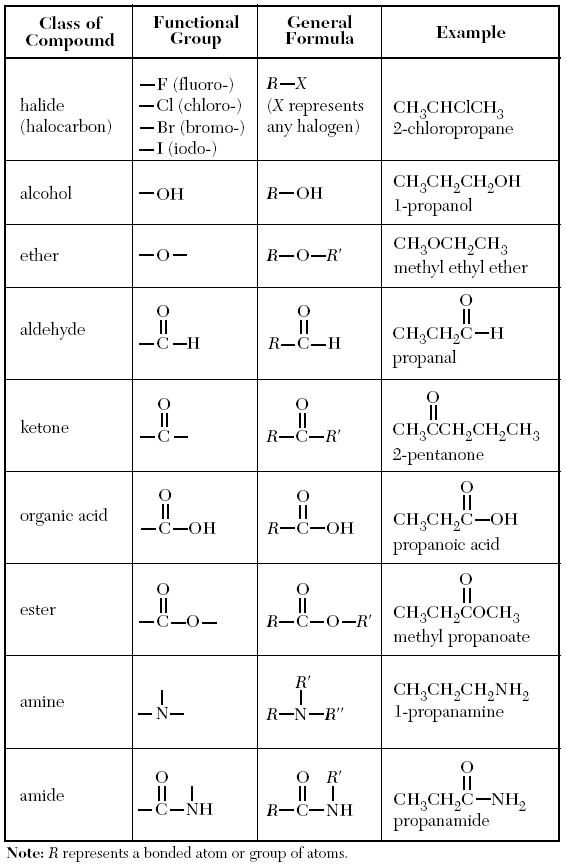
R is like x in math, but you would plug in some sort of Hydrocarbon chain in for it.
Examples of names, and compounds are also shown is the table.
Halide/ Halocarbon → A hydrocarbon attached to any halogen (Group 17 element)
The name ends with however the hydrocarbon is named (Like propane), but for the suffix you would add a the stem of the halogen, and before that the number of the carbon(s) it's attached to.
You could also add di or tri before the halogen stem in the name is there are multiple.
if a methyl, ethyl, or propyl group is also in the molecule, than still add it in the name but AFTER the halogen stem
The stems for certain halogens are given in the table above.
Alcohol → A hydrocarbon group, with a hydroxide (OH) attached to it
To name it, use the prefix for the longest Carbon chain (table P), and end it with “-anol”, still add the number and dash to show which carbon the OH is attached to.
Ether → Two Hydrocarbon chains separated by an Oxygen. (Just has an oxygen in the center of two chains)
The two chains don't have to be identical on either side of the Oxygen.
To name one, there are two names. The common name and the IUPAC name.
IUPAC stands for “International Union of Pure and Applied Chemistry”, they are the ones who come up with naming systems.
For the regents we will focus on the common names.
So you would write the name of the two hydrocarbon groups in alphabetical order, and then end it with “ether”
If the Hydrocarbon group is the same on both sides than use the prefix “di” before writing it.
Aldehyde → A hydrocarbon group attached to a Carbon, which has single bond to a Hydrogen, and a double bond to a Oxygen.
For naming, just use the longest chain of carbons for the prefix, and end it with “-anal”. And the number gets added for the location if needed.
The extra Carbon is included in the longest chain.
Ketone → A carbon in the center which is double bonded to an oxygen, and has two hydrocarbon groups on either side.
For naming count the longest chain again (including the extra Carbon), and end the name with “-anone”, and put the number for the location of the Oxygen brew the name.
Organic Acids → A hydrocarbon group attached to an extra Carbon, which has a single bond with a hydroxide ion, and a double bond with an Oxygen.
For the name use the longest carbon chain prefix, and end it with “-anoic acid”.
Ester → One Hydrocarbon group is attached to a Carbon which is bonded with two Oxygens. one is with a double bond, and the other with a single bond. The single bonded Oxygen is also connected to another hydrocarbon group.
For the first part of the name write down the shortest hydrocarbon group’s name, followed by the longest one, using the longest chain prefix, and end it with “-anoate”
Amine → Three hydrocarbon groups single bonded to on Nitrogen.
Use the longest carbon chain prefix, and end it with “-amine”
Amide → A hydrocarbon group bonded to a Carbon which is double bonded to an Oxygen, and has a single bond to a Nitogen, which is also attached to Hydrogen. On the Nitrogen there is also another hydrocarbon group.
Use the longest carbon chain prefix and it with “-amide”.
Organic Reactions:
→ reactions that deal with Organic compounds. this only goes over the ones relevant to the Regents.
Combustion → A hydrocarbon and Oxygen will burn and create CO
2and H2O.More detail in Unit 5-chemical Reactions
Substitution → When another element like a halogen is being added to a saturated Hydrocarbon (Alkanes), and it replaces one of the Hydrogens, the Hydrogen is then also a product.
In most cases it might be a compound of two of the same element being added, but only one of them will replace the Hydrogen.
The other will then bond with the Hydrogen which was released forming another compound
Example: C
2H6+ Cl2→ C2H5Cl + HCl
Addition → When you add another element to an unsaturated hydrocarbon (Alkenes, & Alkynes), so it will bond to a carbon, where the double or triple bond is. Will break that bond and attach itself instead.
Two or more reagents form one product, similar to synthesis/combination reactions.
Example: C
3H6+ Br2→ C3H6Br2.
Esterification → The formation of an ester, with water as a bi-product.
It is made from an acid, and an alcohol (The reactants)
Saponification → An Ester and a base react to form an ion, and alcohol.
This is how soap is made, and the ions are what gives soap its cleaning qualities.
Fermentation → A carbohydrate such as sugar is broken down into an alcohol and CO
2.How yeast works in baking, and also how alcoholic beverages are made.
Polymerization → When multiple smaller molecules (known as monomers) come together to form a bigger molecule (known as a Polymer). there are two ways this is done
Addition polymers: Polymers that are made by breaking double and triple bonds, to bond the monomers together
Condensation polymers: Taking out water from the monomers allowing them to bond
Cracking → When bigger more complex saturated hydrocarbons are broken down through heat into smaller more useful hydrocarbons.
Next Unit: Nuclear Chemistry
Unit 10- Organic Chemistry
DO NOT CLICK FLASHCARDS FROM HERE (OR STUDY) Click Here____.
* Tables P, Q, and R on the NYS Chemistry Reference Table are used a lot in this unit. And mentioned a lot in these notes.
*Any code blocks ( ) will represent subscripts. Ex: Carbon Dioxide= CO2
Organic Compounds:
→ They all contain Carbon (C), which is the building block of all life on earth. They are Covalent compounds.
In these compounds the carbons make chains which are also bonded to other elements like Nitrogen(N), Oxygen(O), and Hydrogen(H).
Each carbon can ONLY have 4 bonds. due to having 4 valence electrons, leaving 4 bonding sites.
They can be represented by a Molecular formula, like C
3H8.Or by a structural formula (A Lewis structure)

Also a condensed structural formula which writes it out in a line like,
CH
3CH2CH3. Which is the same compound as before (C3H8)This gives some idea as to how a full structural formula for the compound can be drawn.
They can also be written in a stick format where each point of a line represnts a Carbon
The blank spaces would be filled in with hydorgens but arent shown in this notation.
C
3H8would look something like this “/\” becasue the three points are Carbons and the unused bonds would be filled with H.This type of notation most liley wont be used on the NYS Regents.
Characteristics of Organic Compounds:
→ Some characteristics change depending on the compound and its mass/shape. But in general most Organic Compounds share these characteristics in comparison with inorganic compounds (Not containing Carbon)
They are insoluble in water.
most organic compounds are non-polar, and since water is polar it can’t dissolve non-polar compounds. (Like dissolves like)
They do not conduct electricity when dissolved.
They are covalent compounds, and ionic compounds are usually the one which can conduct electricity when dissolved.
They have lower melting points, and boiling points than inorganic compounds
They react a lot slower than inorganic compounds
This is because there is a lot more bond rearranging in Organic compounds compared to Inorganic compounds.
They are also very flammable.
Naming & Functional Groups:
→ When naming organic compounds a lot of the time it comes down to which functional group the compound belongs to. But there are some parts of the name which any organic compounds may share.
Functional Groups → parts of organic molecules which dictate what a certain compound can do
The prefix is linked to the longest un-broken chain of carbons in the compound. These Carbons don’t need to be in a straight line, just an unbroken chain
From there you can use table P on the reference table to find the prefix based on the number.

if there is a number followed by a dash before the compound name, it shows where a certain piece of the compound is located. (Will make sense with more examples later on)
“#-name”
From there, the rest of the name depends on the functional group.
Hydrocarbons:
→ This functional group is made of compounds which ONLY contain Carbon and Hydrogen. This is one of the more important groups and has a lot of rules when it comes to naming. And Hydrocarbons Are usually parts of other functional groups.
As given on Table Q, the Homologous series of hydrocarbons gives a lot of information on the three types of Hydrocarbons and how they work.
Alkanes → These are Hydrocarbon where there are only single bonds connecting the C’s
to find the number of Hydrogens just plug in the number of C’s in to “n” in the General Formula
H= 2n+2
The name starts with the prefix and ends with “ane” for all alkanes.
#-(pre-fix)ane
They are saturated, which means you can’t add anything else onto the Carbons.
Alkenes → These are Hydrocarbons where at least one C to C bond is a double bond
The equation for the number of Hydrogens in this compound is just: H= 2n
For compounds with three or more C’s, and number followed by a dash is used to show where the double bond is.
It is also sometimes not written if the double bond is on the first carbon.
If it is though the number out front would just be 1.
For example the compound name “3-pentene” means that on the third carbon there is a double bond. Or think of it as the the third bond is a double bond.
You could the Carbons from left to right OR right to left either way is correct.
all Alkenes end in “ene”
#-(pre-fix)ene
Alkynes → Hydrocarbons where one of the C to C bonds is a triple bond.
To find the number Hydrogen the equation is: H=2n-2
The number and dash out front is the same with Alkenes, but instead reprenstes the location of the triple bond.
All Alkynes end in “yne”
#-(pre-fix)yne
Both Alkenes, and Alkynes are unsaturated, meaning you could add more elements onto them.
So to name hydrocarbons you must know which type it is.
Examples:
A hydrocarbon with the longest carbon chain being 4, and having a double bond attached to the second carbon would be named: (How many hydrogens? with only 4 carbons)
2-butene
H= 2n= 2(4)= 8
A hydrocarbon which only has single bond and whose longest carbon chain is 8 would be named:(How many hydrogens? with only 8 carbons)
octane
H= 2n+2 = 2(8)+2= 18
A hydrocarbon with a triple bond on the 4th Carbon, with the longest chain of Carbons being 9 would be named: (How many hydrogens? with only 9 carbons)
4-nonyne
H= 2n-2=2(9)-2= 16
Methyl, Ethyl, and Propyl groups:
→ These are not functional groups, but they are Hydrocarbon groups which can attach to a Carbon in an organic compound. You must memorize these.
Methyl groups → A CH
3tacked onto a carbonMeth-, is the prefix for "one” b/c there is only one extra carbon for this group.
In the name of a compound you put a number followed by a dash behind the name ethyl, and then you write the name of the rest the compound after that.
The number represents the carbon where the ethyl group is attached.
An example would be 2-methyl butane.
If there are 2 ethyl groups in the same compound you use the word diethyl
then you write two numbers in front to show which to carbons the ethyls are attached to.
An example would be 3,4- methyl nonane, or if they are on the same carbon 3,3-methyl nonane.
Ethyl groups → A CH
2CH3/ C2H5attached onto a carbon.Eth-, means 2, so 2 carbons are attached.
Same rules for naming just with the word Ethyl instead of methyl.
Examples are: 2-ethyl pentane, or 2, 3-ethyl hexane, or 3, 3- ethyl nonane
Propyl Groups → A CH
2CH2CH3/ C3H7attachedprop-, means 3, so 3 extra carbons
Again same rules for naming.
If one of these is attached to a alkene or alkyne, you must add the number for where the double or triple bond is ASWELL AS the number of the methyl, ethyl, or propyl group location.
For Example: 2-methyl 3-hexene
Isomers:
→ Compounds with the same molecular formula, but different structures/ shapes.
Often these are made with the use of methyl, ethyl, and propyl groups.
For example an isomer of pentane would be 2-methyl butane
They both have a the same molecular formula (C5H12), but different structural formulas)
2-methyl butane could also be called isopentane in this case
Most compounds have multiple isomers, so just giving them their name with methyl, ethyl, or propyl is easier than keeping track of prefixes.
Other Functional Groups:
→ On table R there is a list of functional groups which the regents could ask about, and how they generally look.

R is like x in math, but you would plug in some sort of Hydrocarbon chain in for it.
Examples of names, and compounds are also shown is the table.
Halide/ Halocarbon → A hydrocarbon attached to any halogen (Group 17 element)
The name ends with however the hydrocarbon is named (Like propane), but for the suffix you would add a the stem of the halogen, and before that the number of the carbon(s) it's attached to.
You could also add di or tri before the halogen stem in the name is there are multiple.
if a methyl, ethyl, or propyl group is also in the molecule, than still add it in the name but AFTER the halogen stem
The stems for certain halogens are given in the table above.
Alcohol → A hydrocarbon group, with a hydroxide (OH) attached to it
To name it, use the prefix for the longest Carbon chain (table P), and end it with “-anol”, still add the number and dash to show which carbon the OH is attached to.
Ether → Two Hydrocarbon chains separated by an Oxygen. (Just has an oxygen in the center of two chains)
The two chains don't have to be identical on either side of the Oxygen.
To name one, there are two names. The common name and the IUPAC name.
IUPAC stands for “International Union of Pure and Applied Chemistry”, they are the ones who come up with naming systems.
For the regents we will focus on the common names.
So you would write the name of the two hydrocarbon groups in alphabetical order, and then end it with “ether”
If the Hydrocarbon group is the same on both sides than use the prefix “di” before writing it.
Aldehyde → A hydrocarbon group attached to a Carbon, which has single bond to a Hydrogen, and a double bond to a Oxygen.
For naming, just use the longest chain of carbons for the prefix, and end it with “-anal”. And the number gets added for the location if needed.
The extra Carbon is included in the longest chain.
Ketone → A carbon in the center which is double bonded to an oxygen, and has two hydrocarbon groups on either side.
For naming count the longest chain again (including the extra Carbon), and end the name with “-anone”, and put the number for the location of the Oxygen brew the name.
Organic Acids → A hydrocarbon group attached to an extra Carbon, which has a single bond with a hydroxide ion, and a double bond with an Oxygen.
For the name use the longest carbon chain prefix, and end it with “-anoic acid”.
Ester → One Hydrocarbon group is attached to a Carbon which is bonded with two Oxygens. one is with a double bond, and the other with a single bond. The single bonded Oxygen is also connected to another hydrocarbon group.
For the first part of the name write down the shortest hydrocarbon group’s name, followed by the longest one, using the longest chain prefix, and end it with “-anoate”
Amine → Three hydrocarbon groups single bonded to on Nitrogen.
Use the longest carbon chain prefix, and end it with “-amine”
Amide → A hydrocarbon group bonded to a Carbon which is double bonded to an Oxygen, and has a single bond to a Nitogen, which is also attached to Hydrogen. On the Nitrogen there is also another hydrocarbon group.
Use the longest carbon chain prefix and it with “-amide”.
Organic Reactions:
→ reactions that deal with Organic compounds. this only goes over the ones relevant to the Regents.
Combustion → A hydrocarbon and Oxygen will burn and create CO
2and H2O.More detail in Unit 5-chemical Reactions
Substitution → When another element like a halogen is being added to a saturated Hydrocarbon (Alkanes), and it replaces one of the Hydrogens, the Hydrogen is then also a product.
In most cases it might be a compound of two of the same element being added, but only one of them will replace the Hydrogen.
The other will then bond with the Hydrogen which was released forming another compound
Example: C
2H6+ Cl2→ C2H5Cl + HCl
Addition → When you add another element to an unsaturated hydrocarbon (Alkenes, & Alkynes), so it will bond to a carbon, where the double or triple bond is. Will break that bond and attach itself instead.
Two or more reagents form one product, similar to synthesis/combination reactions.
Example: C
3H6+ Br2→ C3H6Br2.
Esterification → The formation of an ester, with water as a bi-product.
It is made from an acid, and an alcohol (The reactants)
Saponification → An Ester and a base react to form an ion, and alcohol.
This is how soap is made, and the ions are what gives soap its cleaning qualities.
Fermentation → A carbohydrate such as sugar is broken down into an alcohol and CO
2.How yeast works in baking, and also how alcoholic beverages are made.
Polymerization → When multiple smaller molecules (known as monomers) come together to form a bigger molecule (known as a Polymer). there are two ways this is done
Addition polymers: Polymers that are made by breaking double and triple bonds, to bond the monomers together
Condensation polymers: Taking out water from the monomers allowing them to bond
Cracking → When bigger more complex saturated hydrocarbons are broken down through heat into smaller more useful hydrocarbons.
 Knowt
Knowt