
Chapter 11: Atmospheric Pollution
Air pollution is a problem both inside and outdoors.
Human activities have physical, chemical, and biological consequences for the atmosphere.
Key Terms
Acid rain: When nitrogen oxides (NOx) and sulfur oxides (SO2) in the atmosphere mix with the rain, snow, fog, hail, or dust, they can become acid rain and fall to the Earth. This can be in wet or dry forms.
Anthropogenic: Caused by humans.
Carbon dioxide: Traps heat leading to melting ice caps, rising sea levels, and coastal flooding; causes climate change. Can be fixed by carbon sequestration
Carbon monoxide: a colorless, odorless, tasteless gas produced by burning gasoline, wood, propane, charcoal or other fuel.
Improperly ventilated appliances and engines, particularly in a tightly sealed or enclosed space, may allow carbon monoxide to accumulate to dangerous levels.
Catalytic converter: A device that converts pollutants into less dangerous things.
Clean Air Act: American law that regulated the use of lead in fuels and the atmosphere.
Electrostatic precipitators: Remove fine particles like dust, smoke, soot, and ash before they leave the smokestack.
Formaldehyde: colorless, strong-smelling, flammable chemical that is produced industrially and used in building materials such as particleboard, plywood, and other pressed-wood products.
Hydrocarbons: compounds comprised exclusively of carbon and hydrogen and they are by far the dominant components of crude oil, processed petroleum hydrocarbons (gasoline, diesel, kerosene, fuel oil, and lubricating oil), coal tar, creosote, dyestuff, and pyrolysis waste products.
Sulfur dioxide: Makes it difficult to breathe, particularly for people with asthma. Harms trees and plants by damaging leaves and decreasing growth; leads to acid rain. Way to prevent is gas scrubbing or by using fluidized bed combustion.
Thermal inversion: Occurs when pollution gets trapped near the Earth’s surface because of warm air trapping cooler, denser air near the Earth.
Toxic metals: Type of metal decides the toxic effects. Way of prevention is catalytic reduction, electrostatic precipitators, and other technologies.
Lead: Leads to permanent nerve damage, anemia, or mental retardation.
Nitric acid: a colorless liquid with yellow or red fumes with an acrid odor. Exposure to nitric acid can cause irritation to the eyes, skin, and mucous membrane; it can also cause delayed pulmonary edema, pneumonitis, bronchitis, and dental erosion.
Nitrogen oxide: Photochemical smog because of the formation of ozone molecules; cause acid rain. Can cause chronic lung disease and damage to the respiratory system.
Noise pollution: Sound that is loud and long enough to cause harm to humans and animals.
Ozone: Photochemical smog can cause ground-level ozone to occur particularly in the afternoon when it gets hot; can cause a variety of health effects such as chest pain, throat irritation, coughing, and congestion.
Particulates: Can get into the lungs; some are even small enough to get into the bloodstream.
Photochemical smog: This type of smog occurs in warm places that have a lot of people, cars, factories, power plants, etc.
Primary air pollutants: an air pollutant emitted directly from a source.
Radon-222: A gas that comes from uranium decay and is the second leading cause of cancer in America.
Secondary air pollutants: not directly emitted as such, but forms when other pollutants (primary pollutants) react in the atmosphere.
Vapor recovery nozzle: A device added to gasoline fuel pumps that traps the vapors before they can be released to the atmosphere.
Volatile organic hydrocarbons: When they mix with nitrogen oxides and heat they can form photochemical smog.
Wet and dry scrubbers: Air pollution devices that remove particulates so they don’t get into the atmosphere.
Introduction to Air Pollution
When coal combusts, or burns, carbon dioxide, sulfur dioxide, nitrogen oxides, toxic metals, and particulates are released.
These go into the air and can result in both environmental and human health concerns
Carbon dioxide (CO2) traps heat, leading to climate change, melting ice caps, rising sea levels, and coastal flooding.
Carbon dioxide can be removed from the atmosphere through a few processes, but the main one is carbon sequestration.
This is the process of capturing and storing atmospheric carbon dioxide in either rock formations or through terrestrial and aquatic ecosystems.
Sulfur dioxide (SO2) can harm trees and plants by damaging leaves and decreasing growth when the SO2leads to acid rain.
Human health is impacted by sulfur dioxide by making it difficult to breathe, particularly for people with asthma, or other respiratory health concerns.
Sulfur dioxide can be removed by gas scrubbing or by using fluidized bed combustion from coal-fired power plants.
Nitrogen oxides come from a mixture of NOx and NO2. They are colorless, acidic when combined with water, and highly corrosive.
They contribute to the formation of photochemical smog (to be covered in the next section) and to the formation of tropospheric ozone, another component of photochemical smog.
They can form a secondary pollutant, nitric acid, which contributes to acid deposition.
Nitrogen oxides can be removed during burning of fossil fuels by catalytic converters in vehicles. In manufacturing plants, they can be removed by H2O2 or sodium hydroxide scrubbers.
Catalytic converters remove pollutants using redox reactions that convert about 98 percent of the fumes from a car into less harmful gases and scrubbers remove it by spraying the gas through a liquid substance.
There are many toxic metals that can be released from burning coal. These include things like lead, mercury, nickel, tin, cadmium, and arsenic.
The type of toxic metal will determine the environmental and human health effects.
We can limit the amount of toxic metals by using catalytic reduction, electrostatic precipitators, and other technologies to address these pollutants.
Particulates are simply small solid or liquid particles that are suspended in the air. These include things like dust, dirt, soot, and smoke.
The size of the particle can determine the harm they can do to humans. They can get into the lungs and some are even small enough to get in the bloodstream.
They can cause haze and, depending on what the particle is, can acidify lakes and streams, deplete the soil of nutrients, and damage materials.
Particulates can be prevented by using baghouse filters or electrostatic precipitators.
In the United States, a law was passed called the Clean Air Act that has helped control air pollution.
The Environmental Protection Agency, the enforcer of the Clean Air Act, has eliminated lead from gasoline, protecting us from lead poisoning.
Lead poisoning leads to permanent nerve damage, anemia, or mental retardation, and is particularly dangerous for children since their nervous system is still developing.
Primary air pollutants come directly from a source, say your car.
Primary pollutants include sulfur dioxide, carbon monoxide, nitrogen oxides, and particulate matter.
Secondary air pollutants form when two or more primary air pollutants react in the atmosphere.
Examples of secondary pollutants are photochemical smog, ozone, and secondary particulate matter.
Photochemical Smog
Photochemical smog occurs in sunny places that have a lot of people, cars, factories, and power plants.
It is formed when volatile organic hydrocarbons and nitrogen oxides, along with heat and sunlight, mix and pollution occurs.
The major contributors are coal-burning power plants and cars.
This is worse in the summer because of the long hours of sunlight.
It usually forms in the morning as people are driving to work and can be affected by rainfall, wind, daily temperatures, and topography.
Photochemical smog can cause eye irritation and respiratory illness.
In the morning, nitrogen oxide is high because of so many cars on the road; we discussed the problems with this in the prior section.
In the afternoon when it gets hot and the sunlight is the most intense, ozone is produced.
Ozone can cause a variety of health effects such as chest pain, throat irritation, coughing, and congestion.
It is particularly dangerous to people with asthma, bronchitis, and emphysema.
Volatile organic compounds (VOCs) come from many things.
They are up to 10 times higher inside than outside and can come from paints, fuels, formaldehyde, gasoline, varnishes, and wax.
Trees can also be a natural source of these. They can cause eye, nose, and throat irritation as well as headaches and nausea.
Symptoms depends on the exposure level and the health of the person. We can reduce photochemical smog by reducing VOCs and nitrogen oxide.
Thermal Inversion
If you look at Figure 11.1, you can see what happens during a thermal inversion.
This is a weather condition when warm, less dense air moves over dense, cold air.
Because of the thermal inversion and the dense air, pollution, particularly smog and particulates, gets trapped near the ground and can’t “escape” to space.
Normally, the temperature would get colder as you go away from the Earth.
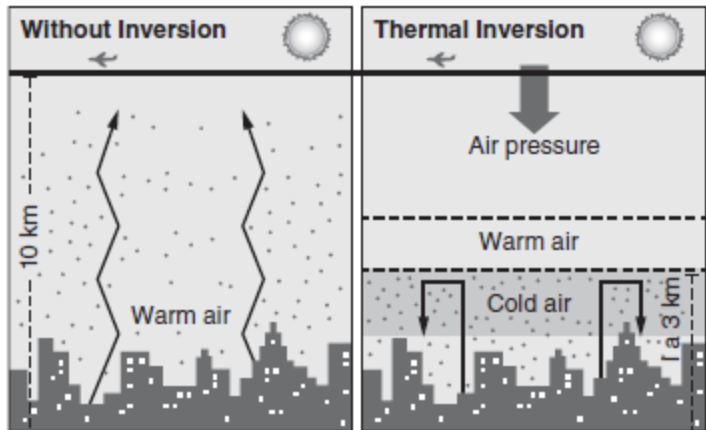
Warm air above the polluted cold air traps the particles and creates poor air quality. This often occurs in regions surrounded by mountains.
Atmospheric CO2 and Particulates
There are many natural forms of CO2 and particulates found on Earth.
For example, every time you exhale, you exhale CO2 and add it to the atmosphere.
CO2 is also formed as things decompose and when volcanoes erupt.
CO2 is measured in parts per million (ppm).
Natural sources of particulates are things like dust, sea salt, pollen, and also things from volcanic eruptions.
Indoor Air Pollutants
Indoor air pollutants come from natural sources; from man-made things like furniture, carpet, and paneling; or from burning things like wood or cigarettes.
Another natural source is radon-222, which comes from the decay of uranium that is found in the ground. This is a particularly dangerous indoor air pollutant because radon decays quickly and when it does tiny radioactive particles are released.
When these are inhaled they can damage the lining of the lungs.
Long-term exposure can lead to lung cancer. When we build homes, and particularly homes with basements where we dig down into the rock or soil, radon gas can work its way into the home via cracks.
It can also enter homes through the groundwater from a local well.
Reduction of Air Pollutants
Catalytic converters are used on automobiles, electrical generators, locomotives, and so on and they take pollutants like carbon monoxide, NOx, and hydrocarbons and make them less harmful.
Scrubbers remove particulates so they don’t get into the atmosphere.
Vapor recovery nozzles are put on gasoline pumps to capture the fumes that are produced.
Electrostatic precipitators remove fine particles like dust, smoke, soot, and ash before they leave the coal-burning smokestack.
Acid Rain (Acid Deposition)
Coal-burning power plants, vehicles, manufacturing, and even volcanoes release nitrogen oxides (NOx) and sulfur oxides (SO2) into the atmosphere. When these chemicals go into the atmosphere and mix with atmospheric water like rain, snow, fog, hail, or dust, they can become acid rain and fall to Earth.
This can be in wet or dry forms.
Winds can then blow this far away from the source, so usually places downwind from the pollution have the biggest problems.
Because acid rain comes from things that humans are doing such as burning coal and driving vehicles, acid rain is considered an anthropogenic pollutant.
Anthropogenic means “caused by human activity.”
When soil or water becomes acidified because of acid deposition there can be some environmental effects.
For example, often you will see dead or dying trees in areas affected by acid rain.
The soil may have higher levels of aluminum in it since acid rain can leach aluminum from the soil; this is toxic to plants and animals. In aquatic biomes acid rain can be harmful to fish and other wildlife.
The aluminum that was leached from the soil on the land may run off into the water and lead to a loss of biodiversity.
The impact on the environment from acid rain depends on the type of soil and rock that is found in the area.
For example, limestone and dolomite have a natural ability to neutralize acid because of the high amounts of calcium carbonate.
Calcium carbonate helps to maintain a constant pH as the minerals react with the acid rain.
One way to remediate the problem is to add limestone (lime) to the soil; lime is a base that counters the effects of the acidic soils.
Noise Pollution
The definition of noise pollution is “harmful or annoying levels of noise, as from airplanes, industry, etc.”
Noise pollution can be high enough to cause damage to people’s hearing and can cause anxiety, poor concentration, loss of productivity, difficulty communicating, lack of sleep, and stress.
It can also cause problems to animals by altering their behavior, making them relocate to quieter places, and impacting migration routes.
It can also impair their ability to navigate, communicate, and reproduce. In marine ecosystems, animals can be impacted because of boats, sonar, and oil and gas drilling.
Chapter 11: Atmospheric Pollution
Air pollution is a problem both inside and outdoors.
Human activities have physical, chemical, and biological consequences for the atmosphere.
Key Terms
Acid rain: When nitrogen oxides (NOx) and sulfur oxides (SO2) in the atmosphere mix with the rain, snow, fog, hail, or dust, they can become acid rain and fall to the Earth. This can be in wet or dry forms.
Anthropogenic: Caused by humans.
Carbon dioxide: Traps heat leading to melting ice caps, rising sea levels, and coastal flooding; causes climate change. Can be fixed by carbon sequestration
Carbon monoxide: a colorless, odorless, tasteless gas produced by burning gasoline, wood, propane, charcoal or other fuel.
Improperly ventilated appliances and engines, particularly in a tightly sealed or enclosed space, may allow carbon monoxide to accumulate to dangerous levels.
Catalytic converter: A device that converts pollutants into less dangerous things.
Clean Air Act: American law that regulated the use of lead in fuels and the atmosphere.
Electrostatic precipitators: Remove fine particles like dust, smoke, soot, and ash before they leave the smokestack.
Formaldehyde: colorless, strong-smelling, flammable chemical that is produced industrially and used in building materials such as particleboard, plywood, and other pressed-wood products.
Hydrocarbons: compounds comprised exclusively of carbon and hydrogen and they are by far the dominant components of crude oil, processed petroleum hydrocarbons (gasoline, diesel, kerosene, fuel oil, and lubricating oil), coal tar, creosote, dyestuff, and pyrolysis waste products.
Sulfur dioxide: Makes it difficult to breathe, particularly for people with asthma. Harms trees and plants by damaging leaves and decreasing growth; leads to acid rain. Way to prevent is gas scrubbing or by using fluidized bed combustion.
Thermal inversion: Occurs when pollution gets trapped near the Earth’s surface because of warm air trapping cooler, denser air near the Earth.
Toxic metals: Type of metal decides the toxic effects. Way of prevention is catalytic reduction, electrostatic precipitators, and other technologies.
Lead: Leads to permanent nerve damage, anemia, or mental retardation.
Nitric acid: a colorless liquid with yellow or red fumes with an acrid odor. Exposure to nitric acid can cause irritation to the eyes, skin, and mucous membrane; it can also cause delayed pulmonary edema, pneumonitis, bronchitis, and dental erosion.
Nitrogen oxide: Photochemical smog because of the formation of ozone molecules; cause acid rain. Can cause chronic lung disease and damage to the respiratory system.
Noise pollution: Sound that is loud and long enough to cause harm to humans and animals.
Ozone: Photochemical smog can cause ground-level ozone to occur particularly in the afternoon when it gets hot; can cause a variety of health effects such as chest pain, throat irritation, coughing, and congestion.
Particulates: Can get into the lungs; some are even small enough to get into the bloodstream.
Photochemical smog: This type of smog occurs in warm places that have a lot of people, cars, factories, power plants, etc.
Primary air pollutants: an air pollutant emitted directly from a source.
Radon-222: A gas that comes from uranium decay and is the second leading cause of cancer in America.
Secondary air pollutants: not directly emitted as such, but forms when other pollutants (primary pollutants) react in the atmosphere.
Vapor recovery nozzle: A device added to gasoline fuel pumps that traps the vapors before they can be released to the atmosphere.
Volatile organic hydrocarbons: When they mix with nitrogen oxides and heat they can form photochemical smog.
Wet and dry scrubbers: Air pollution devices that remove particulates so they don’t get into the atmosphere.
Introduction to Air Pollution
When coal combusts, or burns, carbon dioxide, sulfur dioxide, nitrogen oxides, toxic metals, and particulates are released.
These go into the air and can result in both environmental and human health concerns
Carbon dioxide (CO2) traps heat, leading to climate change, melting ice caps, rising sea levels, and coastal flooding.
Carbon dioxide can be removed from the atmosphere through a few processes, but the main one is carbon sequestration.
This is the process of capturing and storing atmospheric carbon dioxide in either rock formations or through terrestrial and aquatic ecosystems.
Sulfur dioxide (SO2) can harm trees and plants by damaging leaves and decreasing growth when the SO2leads to acid rain.
Human health is impacted by sulfur dioxide by making it difficult to breathe, particularly for people with asthma, or other respiratory health concerns.
Sulfur dioxide can be removed by gas scrubbing or by using fluidized bed combustion from coal-fired power plants.
Nitrogen oxides come from a mixture of NOx and NO2. They are colorless, acidic when combined with water, and highly corrosive.
They contribute to the formation of photochemical smog (to be covered in the next section) and to the formation of tropospheric ozone, another component of photochemical smog.
They can form a secondary pollutant, nitric acid, which contributes to acid deposition.
Nitrogen oxides can be removed during burning of fossil fuels by catalytic converters in vehicles. In manufacturing plants, they can be removed by H2O2 or sodium hydroxide scrubbers.
Catalytic converters remove pollutants using redox reactions that convert about 98 percent of the fumes from a car into less harmful gases and scrubbers remove it by spraying the gas through a liquid substance.
There are many toxic metals that can be released from burning coal. These include things like lead, mercury, nickel, tin, cadmium, and arsenic.
The type of toxic metal will determine the environmental and human health effects.
We can limit the amount of toxic metals by using catalytic reduction, electrostatic precipitators, and other technologies to address these pollutants.
Particulates are simply small solid or liquid particles that are suspended in the air. These include things like dust, dirt, soot, and smoke.
The size of the particle can determine the harm they can do to humans. They can get into the lungs and some are even small enough to get in the bloodstream.
They can cause haze and, depending on what the particle is, can acidify lakes and streams, deplete the soil of nutrients, and damage materials.
Particulates can be prevented by using baghouse filters or electrostatic precipitators.
In the United States, a law was passed called the Clean Air Act that has helped control air pollution.
The Environmental Protection Agency, the enforcer of the Clean Air Act, has eliminated lead from gasoline, protecting us from lead poisoning.
Lead poisoning leads to permanent nerve damage, anemia, or mental retardation, and is particularly dangerous for children since their nervous system is still developing.
Primary air pollutants come directly from a source, say your car.
Primary pollutants include sulfur dioxide, carbon monoxide, nitrogen oxides, and particulate matter.
Secondary air pollutants form when two or more primary air pollutants react in the atmosphere.
Examples of secondary pollutants are photochemical smog, ozone, and secondary particulate matter.
Photochemical Smog
Photochemical smog occurs in sunny places that have a lot of people, cars, factories, and power plants.
It is formed when volatile organic hydrocarbons and nitrogen oxides, along with heat and sunlight, mix and pollution occurs.
The major contributors are coal-burning power plants and cars.
This is worse in the summer because of the long hours of sunlight.
It usually forms in the morning as people are driving to work and can be affected by rainfall, wind, daily temperatures, and topography.
Photochemical smog can cause eye irritation and respiratory illness.
In the morning, nitrogen oxide is high because of so many cars on the road; we discussed the problems with this in the prior section.
In the afternoon when it gets hot and the sunlight is the most intense, ozone is produced.
Ozone can cause a variety of health effects such as chest pain, throat irritation, coughing, and congestion.
It is particularly dangerous to people with asthma, bronchitis, and emphysema.
Volatile organic compounds (VOCs) come from many things.
They are up to 10 times higher inside than outside and can come from paints, fuels, formaldehyde, gasoline, varnishes, and wax.
Trees can also be a natural source of these. They can cause eye, nose, and throat irritation as well as headaches and nausea.
Symptoms depends on the exposure level and the health of the person. We can reduce photochemical smog by reducing VOCs and nitrogen oxide.
Thermal Inversion
If you look at Figure 11.1, you can see what happens during a thermal inversion.
This is a weather condition when warm, less dense air moves over dense, cold air.
Because of the thermal inversion and the dense air, pollution, particularly smog and particulates, gets trapped near the ground and can’t “escape” to space.
Normally, the temperature would get colder as you go away from the Earth.

Warm air above the polluted cold air traps the particles and creates poor air quality. This often occurs in regions surrounded by mountains.
Atmospheric CO2 and Particulates
There are many natural forms of CO2 and particulates found on Earth.
For example, every time you exhale, you exhale CO2 and add it to the atmosphere.
CO2 is also formed as things decompose and when volcanoes erupt.
CO2 is measured in parts per million (ppm).
Natural sources of particulates are things like dust, sea salt, pollen, and also things from volcanic eruptions.
Indoor Air Pollutants
Indoor air pollutants come from natural sources; from man-made things like furniture, carpet, and paneling; or from burning things like wood or cigarettes.
Another natural source is radon-222, which comes from the decay of uranium that is found in the ground. This is a particularly dangerous indoor air pollutant because radon decays quickly and when it does tiny radioactive particles are released.
When these are inhaled they can damage the lining of the lungs.
Long-term exposure can lead to lung cancer. When we build homes, and particularly homes with basements where we dig down into the rock or soil, radon gas can work its way into the home via cracks.
It can also enter homes through the groundwater from a local well.
Reduction of Air Pollutants
Catalytic converters are used on automobiles, electrical generators, locomotives, and so on and they take pollutants like carbon monoxide, NOx, and hydrocarbons and make them less harmful.
Scrubbers remove particulates so they don’t get into the atmosphere.
Vapor recovery nozzles are put on gasoline pumps to capture the fumes that are produced.
Electrostatic precipitators remove fine particles like dust, smoke, soot, and ash before they leave the coal-burning smokestack.
Acid Rain (Acid Deposition)
Coal-burning power plants, vehicles, manufacturing, and even volcanoes release nitrogen oxides (NOx) and sulfur oxides (SO2) into the atmosphere. When these chemicals go into the atmosphere and mix with atmospheric water like rain, snow, fog, hail, or dust, they can become acid rain and fall to Earth.
This can be in wet or dry forms.
Winds can then blow this far away from the source, so usually places downwind from the pollution have the biggest problems.
Because acid rain comes from things that humans are doing such as burning coal and driving vehicles, acid rain is considered an anthropogenic pollutant.
Anthropogenic means “caused by human activity.”
When soil or water becomes acidified because of acid deposition there can be some environmental effects.
For example, often you will see dead or dying trees in areas affected by acid rain.
The soil may have higher levels of aluminum in it since acid rain can leach aluminum from the soil; this is toxic to plants and animals. In aquatic biomes acid rain can be harmful to fish and other wildlife.
The aluminum that was leached from the soil on the land may run off into the water and lead to a loss of biodiversity.
The impact on the environment from acid rain depends on the type of soil and rock that is found in the area.
For example, limestone and dolomite have a natural ability to neutralize acid because of the high amounts of calcium carbonate.
Calcium carbonate helps to maintain a constant pH as the minerals react with the acid rain.
One way to remediate the problem is to add limestone (lime) to the soil; lime is a base that counters the effects of the acidic soils.
Noise Pollution
The definition of noise pollution is “harmful or annoying levels of noise, as from airplanes, industry, etc.”
Noise pollution can be high enough to cause damage to people’s hearing and can cause anxiety, poor concentration, loss of productivity, difficulty communicating, lack of sleep, and stress.
It can also cause problems to animals by altering their behavior, making them relocate to quieter places, and impacting migration routes.
It can also impair their ability to navigate, communicate, and reproduce. In marine ecosystems, animals can be impacted because of boats, sonar, and oil and gas drilling.
 Knowt
Knowt
