
PY 131 Chapter 12/13: Solids and Liquids
Chapter 12
Matter
Matter comes in three common phases: solids, liquids and gases.
Solid (rigid) bodies - have fixed shapes and volumes.
Liquids - do not have fixed shapes but the volume is fixed.
Gases - have neither fixed shapes nor volumes.
Liquids and gases are collectively known as fluids. There are substances that do not fit neatly into this scheme e.g.
glasses, liquid crystals, colloids,
Many substances exhibit two or more solid phases and we also talk about liquid phases (which means they do not mix),
e.g. water (15 solid phases)
Atomic Theory
The idea that matter is made up of atoms – the smallest piece of a substance – dates back to the ancient Greeks and Indians.
the idea was picked up again in the 17th century but it was only in the 18th century that experimental evidence was obtained supporting this theory and not until the 20th that it was completely accepted.
Robert Boyle is widely regarded as the first modern chemist.
His study of alchemy led him to the idea that some substances were made up of atoms of all the same kind.
The first list of elements by Lavoisier included Carbon, Oxygen, Nitrogen, Hydrogen, Sulfur, Phosphorous, 'light' and 'heat'
Dmitri Mendeleev had 66 elements when he made his table.
Substances can be divided into elements and compounds.
Elements are made up of only one type of atom,
Compounds are made up of more than one type.
Molecules are clusters of atoms.
Atoms are very small and light.
it is possible to weigh one atom
Atomic weights are usually reported in atomic mass units (u).
Chemists call this same unit the Dalton (Da).
1 u = 1.6605 x 10-27 kg
The mass of a hydrogen atom is 1.0078 u
Atoms interact with each other:
At ‘long’ distances the interactions result in attractive forces
At ‘short’ distances the interactions are repulsive
The forces between atoms are conservative (they are electric and magnetic in origin) so there is an associated potential energy.
The difference between solids, liquids, and gases, is the relative amount of potential energy compared to the kinetic.
Solids have a lot of potential energy, gases have little.
Moles
The amount (not the mass) of matter is usually given the symbol n and measured in a unit called moles mol.
One mole corresponds to the number of 12C atoms in 12g of pure Carbon-12.
One mole of a substance has a mass called its molar mass M.
The units of molar mass are usually given as g / mol.
Using this definition, the amount of a substance, the number of moles, is its mass m divided by its molar mass.
n= m / M
The molar mass can be found approximately if the chemical formula for the substance is known, from tables or online
molar mass M of CO2≈ 1 x 12 g + 2 x 16 g ≈ 44 g / mol
EXAMPLE 1:
Which has more molecules - a mole of nitrogen (N2 ) gas or a mole of oxygen (O2 ) gas?
They have the same amount of molecules because they each have a mol.
Which weighs more - a mole of nitrogen (N2 ) gas or a mole of oxygen (O2 ) gas?
The Oxygen gas weighs more because it has a higher molecular weight.
Density and Specific Gravity
Particularly for fluids, mass is not the most useful quantity.
The properties of the fluid are better described with the density.
The (mass) density of a substance is the mass per unit volume.
The symbol for mass density is usually the Greek symbol ρ.
ρ= m / V
The SI units of density are kg / m^3.
The specific gravity of a substance is the ratio of its density relative to the density of water at 4°C.
Specific gravity has no units.
The densest material on Earth is metallic Osmium at 22.6 x 103 kg/m3 .
The densest matter in the Universe is the core of a neutron star ~1018 kg/m3 .
The most tenuous matter in the Universe is the Universe ~10-19 kg/m3 , ~0.2 atoms per cubic meter
Chapter 13
Pressure
Fluids exert forces upon the walls of their containers.
But force is not a very useful quantity for describing fluids, the more useful quantity is pressure.
The pressure of a fluid is the size of the force F it exerts perpendicular to a surface per unit area A of the surface.
P= F / A
The SI unit of pressure is the Pascal, Pa or N/m^2.
There are many (many) other units: psi, mm-Hg, bar, atmospheres
Pressure and force are related but are different: pressure is a scalar, not a vector. A fluid exerts pressure on everything it is in contact with.
e.g. the air in this room exerts pressure on the ceiling and walls.
The pressure a fluid exerts is different from its weight.
Hydrostatic Pressure
Consider a column of fluid of density ρ, area A.
The pressure in the fluid is not constant with depth.
The forces acting on a slab of fluid are:
the pressure from the fluid above the slab,
the pressure from the fluid below the slab,
the weight of the slab.
In typical situations, the pressure at the top of the fluid is not zero e.g. a liquid is open to the atmosphere.
If the atmospheric pressure is P0 and h is the depth below the surface, the pressure at that depth is
P=P0+ ρgh
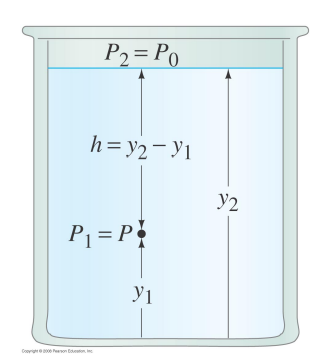
The pressure only depends upon the vertical distance to the surface, not on the amount of fluid in a container or shape.

Consider three containers filled with water to the same depth.
If the surface area at the base of each container is the same, the force of the fluid upon the base is the same.
The mass and weight of the water is different for each.
depending upon the shape of the container, the force from the fluid pressure can be less than or greater than the weight.
Pascal's Principle
Pascal's principle (or law) describes how pressure in a fluid is transmitted through a fluid.
If an external pressure is applied to a confined fluid, the pressure at every point within the fluid increases by that amount.
This principle is used in many devices because it allows small forces to be turned into big forces.
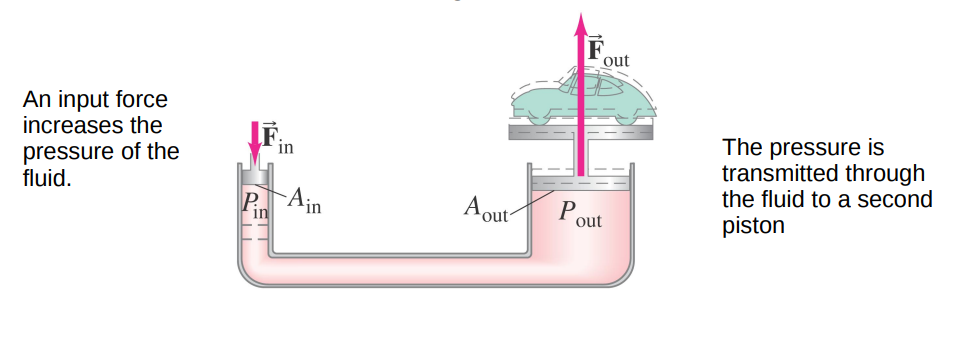
According to Pascal's principle
P_out =P_in
F_out / A_out =F_in / A_in
F_out=F_in (A_out / A_in)
If the ratio of the piston areas is large then F_out can be much greater than F_in.
As with a lever, the ratio of F_out to F_in is called the mechanical advantage.
Buoyancy
Objects immersed in a fluid appear to weigh less than in a vacuum
Some objects float – they appear to have negative weight!
The reason for both effects is because of a buoyancy force exerted by the liquid upon the object.
This buoyancy force is the net force on an object from the fluid pressure.
Consider an object of volume V in a fluid with density ρF .

The buoyancy force, which points upward, is equal to the weight of fluid displaced by the object.
This result is known as Archimedes' principle and is valid for all objects, no matter their shape.
Archimedes' principle can be used to find the density of any object.
first, weigh the object in a vacuum (air if you don’t have a vacuum handy),
second weigh the object in a fluid,
the difference is the weight of the fluid displaced by the object,
and the volume of the object is the volume of the displaced fluid.
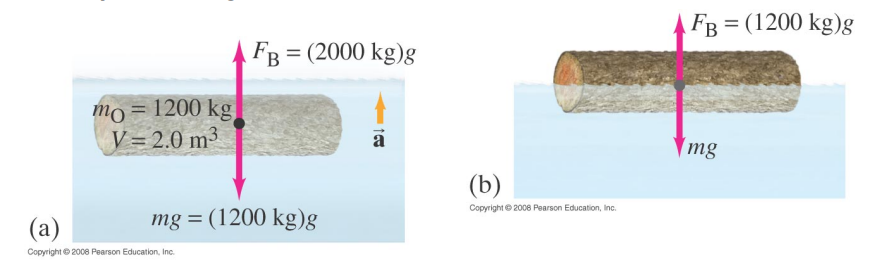
Objects float because the density of the object is less than that of the displaced fluid
Fully immersed, the buoyancy force upwards is greater than the object’s weight downwards.
Only upon the surface when a fraction of the object’s volume displaces the fluid is the buoyancy force is equal to the object’s weight.
EXAMPLE 1
A boat carrying a large chunk of steel is floating on a lake. The chunk is then thrown overboard and sinks. What happens to the water level in the lake (with respect to the shore)?
The water level in the lake will go down when the chunk of steel is thrown overboard and sinks. This is because the chunk of steel displaces its weight in water when it is floating, but when it sinks, it displaces its weight in air, which is less than its weight in water. Therefore, the water level in the lake decreases as the chunk of steel sinks.
EXAMPLE 2
A block of wood floats half-in half-out in a container of water. If the same setup was moved to the Moon, what would be the new level of the block?
The level of the block would still be half-in half-out of the water in the container on the Moon. This is because the buoyancy force acting on the block depends on the weight of the displaced fluid, which is determined by the volume of the block that is submerged in the water, and is not affected by the strength of the gravitational field. The weight of the block and the water it displaces may be different on the Moon due to the lower gravity, but this does not affect the level of the block in the water.
EXAMPLE 3
A block floats in water with 3/4 of its volume submerged. The water is then replaced with oil which has a density half that of water. Is more or less of the block submerged now?
When a block floats in water with 3/4 of its volume submerged, it means that the weight of the water displaced by the block is equal to the weight of the block. When the water is replaced with oil, the weight of the oil displaced by the block will be equal to the weight of the block, since the block hasn't changed.
Since the density of the oil is half that of water, the weight of the oil displaced by the block will be twice that of the water displaced by the block. Therefore, the block will displace more oil than water and more of the block will be submerged in the oil.
In other words, more of the block will be submerged when it is placed in the oil.
EXAMPLE 4
A block floats in water with 3/4 of its volume submerged. Oil, with a density half that of water, is then poured on top. What happens to the volume of the block in the water (i.e. does it increase, or decrease)?
The volume of the block submerged in water would decrease if oil, with a density half that of water, is poured on top.
This is because the addition of oil on top of the water would create a new interface with a lower pressure compared to the water alone, and this would cause the block to float higher. As the block floats higher, it displaces less water, and therefore less of its volume is submerged.
PY 131 Chapter 12/13: Solids and Liquids
Chapter 12
Matter
Matter comes in three common phases: solids, liquids and gases.
Solid (rigid) bodies - have fixed shapes and volumes.
Liquids - do not have fixed shapes but the volume is fixed.
Gases - have neither fixed shapes nor volumes.
Liquids and gases are collectively known as fluids. There are substances that do not fit neatly into this scheme e.g.
glasses, liquid crystals, colloids,
Many substances exhibit two or more solid phases and we also talk about liquid phases (which means they do not mix),
e.g. water (15 solid phases)
Atomic Theory
The idea that matter is made up of atoms – the smallest piece of a substance – dates back to the ancient Greeks and Indians.
the idea was picked up again in the 17th century but it was only in the 18th century that experimental evidence was obtained supporting this theory and not until the 20th that it was completely accepted.
Robert Boyle is widely regarded as the first modern chemist.
His study of alchemy led him to the idea that some substances were made up of atoms of all the same kind.
The first list of elements by Lavoisier included Carbon, Oxygen, Nitrogen, Hydrogen, Sulfur, Phosphorous, 'light' and 'heat'
Dmitri Mendeleev had 66 elements when he made his table.
Substances can be divided into elements and compounds.
Elements are made up of only one type of atom,
Compounds are made up of more than one type.
Molecules are clusters of atoms.
Atoms are very small and light.
it is possible to weigh one atom
Atomic weights are usually reported in atomic mass units (u).
Chemists call this same unit the Dalton (Da).
1 u = 1.6605 x 10-27 kg
The mass of a hydrogen atom is 1.0078 u

Atoms interact with each other:
At ‘long’ distances the interactions result in attractive forces
At ‘short’ distances the interactions are repulsive
The forces between atoms are conservative (they are electric and magnetic in origin) so there is an associated potential energy.
The difference between solids, liquids, and gases, is the relative amount of potential energy compared to the kinetic.
Solids have a lot of potential energy, gases have little.
Moles
The amount (not the mass) of matter is usually given the symbol n and measured in a unit called moles mol.
One mole corresponds to the number of 12C atoms in 12g of pure Carbon-12.
One mole of a substance has a mass called its molar mass M.
The units of molar mass are usually given as g / mol.
Using this definition, the amount of a substance, the number of moles, is its mass m divided by its molar mass.
n= m / M
The molar mass can be found approximately if the chemical formula for the substance is known, from tables or online
molar mass M of CO2≈ 1 x 12 g + 2 x 16 g ≈ 44 g / mol
EXAMPLE 1:
Which has more molecules - a mole of nitrogen (N2 ) gas or a mole of oxygen (O2 ) gas?
They have the same amount of molecules because they each have a mol.
Which weighs more - a mole of nitrogen (N2 ) gas or a mole of oxygen (O2 ) gas?
The Oxygen gas weighs more because it has a higher molecular weight.
Density and Specific Gravity
Particularly for fluids, mass is not the most useful quantity.
The properties of the fluid are better described with the density.
The (mass) density of a substance is the mass per unit volume.
The symbol for mass density is usually the Greek symbol ρ.
ρ= m / V
The SI units of density are kg / m^3.
The specific gravity of a substance is the ratio of its density relative to the density of water at 4°C.
Specific gravity has no units.
The densest material on Earth is metallic Osmium at 22.6 x 103 kg/m3 .
The densest matter in the Universe is the core of a neutron star ~1018 kg/m3 .
The most tenuous matter in the Universe is the Universe ~10-19 kg/m3 , ~0.2 atoms per cubic meter
Chapter 13
Pressure
Fluids exert forces upon the walls of their containers.
But force is not a very useful quantity for describing fluids, the more useful quantity is pressure.
The pressure of a fluid is the size of the force F it exerts perpendicular to a surface per unit area A of the surface.
P= F / A
The SI unit of pressure is the Pascal, Pa or N/m^2.
There are many (many) other units: psi, mm-Hg, bar, atmospheres
Pressure and force are related but are different: pressure is a scalar, not a vector. A fluid exerts pressure on everything it is in contact with.
e.g. the air in this room exerts pressure on the ceiling and walls.
The pressure a fluid exerts is different from its weight.
Hydrostatic Pressure
Consider a column of fluid of density ρ, area A.
The pressure in the fluid is not constant with depth.
The forces acting on a slab of fluid are:
the pressure from the fluid above the slab,
the pressure from the fluid below the slab,
the weight of the slab.
In typical situations, the pressure at the top of the fluid is not zero e.g. a liquid is open to the atmosphere.
If the atmospheric pressure is P0 and h is the depth below the surface, the pressure at that depth is
P=P0+ ρgh

The pressure only depends upon the vertical distance to the surface, not on the amount of fluid in a container or shape.

Consider three containers filled with water to the same depth.
If the surface area at the base of each container is the same, the force of the fluid upon the base is the same.
The mass and weight of the water is different for each.
depending upon the shape of the container, the force from the fluid pressure can be less than or greater than the weight.
Pascal's Principle
Pascal's principle (or law) describes how pressure in a fluid is transmitted through a fluid.
If an external pressure is applied to a confined fluid, the pressure at every point within the fluid increases by that amount.
This principle is used in many devices because it allows small forces to be turned into big forces.

According to Pascal's principle
P_out =P_in
F_out / A_out =F_in / A_in
F_out=F_in (A_out / A_in)
If the ratio of the piston areas is large then F_out can be much greater than F_in.
As with a lever, the ratio of F_out to F_in is called the mechanical advantage.
Buoyancy
Objects immersed in a fluid appear to weigh less than in a vacuum
Some objects float – they appear to have negative weight!
The reason for both effects is because of a buoyancy force exerted by the liquid upon the object.
This buoyancy force is the net force on an object from the fluid pressure.
Consider an object of volume V in a fluid with density ρF .

The buoyancy force, which points upward, is equal to the weight of fluid displaced by the object.
This result is known as Archimedes' principle and is valid for all objects, no matter their shape.
Archimedes' principle can be used to find the density of any object.
first, weigh the object in a vacuum (air if you don’t have a vacuum handy),
second weigh the object in a fluid,
the difference is the weight of the fluid displaced by the object,
and the volume of the object is the volume of the displaced fluid.
Objects float because the density of the object is less than that of the displaced fluid
Fully immersed, the buoyancy force upwards is greater than the object’s weight downwards.
Only upon the surface when a fraction of the object’s volume displaces the fluid is the buoyancy force is equal to the object’s weight.

EXAMPLE 1
A boat carrying a large chunk of steel is floating on a lake. The chunk is then thrown overboard and sinks. What happens to the water level in the lake (with respect to the shore)?
The water level in the lake will go down when the chunk of steel is thrown overboard and sinks. This is because the chunk of steel displaces its weight in water when it is floating, but when it sinks, it displaces its weight in air, which is less than its weight in water. Therefore, the water level in the lake decreases as the chunk of steel sinks.
EXAMPLE 2
A block of wood floats half-in half-out in a container of water. If the same setup was moved to the Moon, what would be the new level of the block?
The level of the block would still be half-in half-out of the water in the container on the Moon. This is because the buoyancy force acting on the block depends on the weight of the displaced fluid, which is determined by the volume of the block that is submerged in the water, and is not affected by the strength of the gravitational field. The weight of the block and the water it displaces may be different on the Moon due to the lower gravity, but this does not affect the level of the block in the water.
EXAMPLE 3
A block floats in water with 3/4 of its volume submerged. The water is then replaced with oil which has a density half that of water. Is more or less of the block submerged now?
When a block floats in water with 3/4 of its volume submerged, it means that the weight of the water displaced by the block is equal to the weight of the block. When the water is replaced with oil, the weight of the oil displaced by the block will be equal to the weight of the block, since the block hasn't changed.
Since the density of the oil is half that of water, the weight of the oil displaced by the block will be twice that of the water displaced by the block. Therefore, the block will displace more oil than water and more of the block will be submerged in the oil.
In other words, more of the block will be submerged when it is placed in the oil.
EXAMPLE 4
A block floats in water with 3/4 of its volume submerged. Oil, with a density half that of water, is then poured on top. What happens to the volume of the block in the water (i.e. does it increase, or decrease)?
The volume of the block submerged in water would decrease if oil, with a density half that of water, is poured on top.
This is because the addition of oil on top of the water would create a new interface with a lower pressure compared to the water alone, and this would cause the block to float higher. As the block floats higher, it displaces less water, and therefore less of its volume is submerged.
 Knowt
Knowt
