
Chapter 24- Alcohols and Carboxylic Acids
ALCOHOLS
Alcohols are organic compounds with the hydroxyl functional group and the general formula C
nH2n+2.Alcohols are soluble in water but their solubility decreases as the chain of carbon atoms becomes longer.
Alcohols are neutral in pH and can undergo combustion and oxidation.
The first four alchols are liquids at room temperature and pressure.

In presence of potassium manganate (VII) which is an oxidising agent, alkenes are oxidised to alcohols. Potassium Manganate (VII) changes colour from purple to colourless.
Ethene is oxdised to ethanol which is one way to produce this alcohol.
Another way to produce ethanol is by fermentation of yeast in presence of enzymes, 37 degrees celsius temperature and a neutral (7) pH. When yeast respires anaerobically, glucose is broken into carbon dioxide and ethanol.
Ethanol is used as a solvent, in alcoholic drinks, and as a fuel.
CARBOXYLIC ACIDS
Carboxylic acids are organic acids with the functional group -COOH, and the general formula C
nH2n+1COOH.As the chain of carbon atoms increases, the melting and boiling points of carboxylic acids also increases.
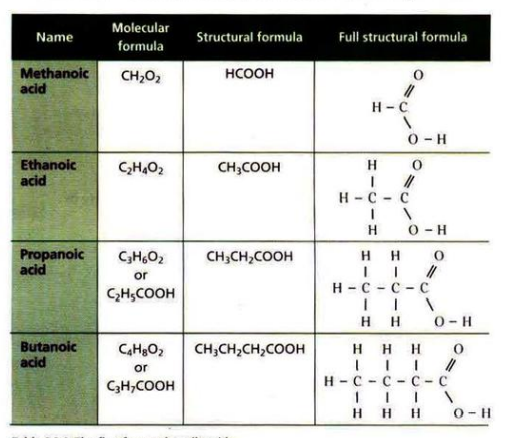
Carboxylic acids undergo the same reactions as normal acids, i.e. with alkalis/bases, carbonates and metals.
They are usually weak acids with pH ranging from 3 to 5.
ESTERS
A carboxylic acid reacts with an alcohol to form an organic compound called ester in a process called esterification.
The first part of the name of the ester is the name of the alcohol with -yl at the end. The second part is the name of the carboxylic acid with -oate at the end. For e.g. ethyl methanoate is made from ethanol and methanoic acid.
Esters are found in fats and waxes.
They are used as solvents, in perfumes, and food flavourings.
Chapter 24- Alcohols and Carboxylic Acids
ALCOHOLS
Alcohols are organic compounds with the hydroxyl functional group and the general formula C
nH2n+2.Alcohols are soluble in water but their solubility decreases as the chain of carbon atoms becomes longer.
Alcohols are neutral in pH and can undergo combustion and oxidation.
The first four alchols are liquids at room temperature and pressure.

In presence of potassium manganate (VII) which is an oxidising agent, alkenes are oxidised to alcohols. Potassium Manganate (VII) changes colour from purple to colourless.
Ethene is oxdised to ethanol which is one way to produce this alcohol.
Another way to produce ethanol is by fermentation of yeast in presence of enzymes, 37 degrees celsius temperature and a neutral (7) pH. When yeast respires anaerobically, glucose is broken into carbon dioxide and ethanol.
Ethanol is used as a solvent, in alcoholic drinks, and as a fuel.
CARBOXYLIC ACIDS
Carboxylic acids are organic acids with the functional group -COOH, and the general formula C
nH2n+1COOH.As the chain of carbon atoms increases, the melting and boiling points of carboxylic acids also increases.

Carboxylic acids undergo the same reactions as normal acids, i.e. with alkalis/bases, carbonates and metals.
They are usually weak acids with pH ranging from 3 to 5.
ESTERS
A carboxylic acid reacts with an alcohol to form an organic compound called ester in a process called esterification.
The first part of the name of the ester is the name of the alcohol with -yl at the end. The second part is the name of the carboxylic acid with -oate at the end. For e.g. ethyl methanoate is made from ethanol and methanoic acid.
Esters are found in fats and waxes.
They are used as solvents, in perfumes, and food flavourings.
 Knowt
Knowt
