Unit 11- Nuclear Chemistry
DO NOT CLICK FLASHCARDS FROM HERE (OR STUDY) Click Here
* Tables N, and O, as well as the Periodic Table of Elements from the NYS Chemistry Reference table are very relevant to this unit.
*This is the last Unit for TOW chemistry
The Nucleus and Transmutation:
*Nuclear chemistry deals with Nuclei, and how they decay once they get too big and unstable.
Unstable Nuclei →they decay and emit particles due to the fact that they are so large. They contain lots of Protons and Neutrons, making them very heavy.
Lots of Energy is used in the Nucleus to keep all of the protons together, since they have the same charge and really don’t like to be shoved in the same nucleus.
The more protons there are the more unstable the Nucleus is.
Nuclei just mean more than one Nucleus.
When a Nucleus is unstable it will decay in a process known as Transmutation.
Transmutation → When the Nucleus of an Atom changes, changing the element.
When this happen the atom will release a radioactive particle, and the leftover Nucleus will become a different atom due to a different number of protons.
Energy is also released, as less energy is needed to hold less protons together.
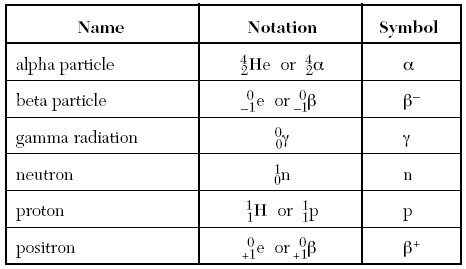
The particles which can be released are shown on Table O.
The Number at the top left of the symbol is the mass (Protons + Neutrons)
The Mass of an alpha particle/ Helium atom is 4.
The Number below that one is the number of protons/ charge
The number of protons in an alpha particle is 2.
Penetration Power →The ability for a particle to pass through matter
Gamma radiation has the greatest penetrating power, since it has no mass or charge making it easier to pass through matter
Often in reaction gamma is omitted because it’s just energy, and dosent take much from the Nucleus.
Transmutation Reactions:
* These reactions show how Transmutation occurs, but there are two types (Artificial and Natural)
Natural Transmutation →When an Unstable Nuclei decays on it’s own in nature. Usually will happen with the half-life.
An equation will have one reactant (left side), and 2 products (right side)
Artificial Transmutation →When humans shoot a particle at an unstable nuclei causing it to break apart. picture it as like a laser zapping off a piece of the nucleus.
An equation will have 2 reactants and 2 products.
For the Equations the mass must be equal, and the number of protons total must be equal on both sides.
If in a natural Transmutation reaction, the reactant has a mass of 20, and has 7 protons, than the sum of the mass on the products must equal 20, and the sum of the protons must also equal 7.
Same with the sums of an artificial Transmutation reaction.
Examples:
Artificial Transmutation:
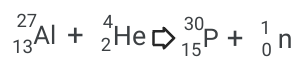
The sum of the top numbers(mass) is the same on both sides (31)
The sum of the bottom numbers (Protons) is also the same on both sides (15)
Aluminum is being bombarded with Helium (which can also be called an alpha particle.)
This releases a neutron when it hits the nucleus.
Natural Transmutation:
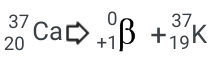
The sum of the mass for the products equals 27, and the sum of the protons equals 20
Ca is naturally decaying, and releases a positron, and made Potassium with the leftover Protons and neutrons.
Fission and Fusion:
Fusion → two lighter Nuclei combine to form one heavier nucleus with a release of energy.
What the sun does
Will usually involve Hydrogen and Helium
Two light atoms like H or He, will combine to form either a H or He with a heavier mass/ nucleus
Fission → Forcing a radioactive particle to collide with a large radioactive element, causing it to split apart and release lots of stored energy
Artificial Transmutation
What is done at Nuclear power plants.
One atom is split into TWO new elements rather than one.
Half-Lives:
→ The time it takes for a radioactive isotope to lose half of its mass.
It loses the mass due to natural transmuation and decay.
Table N, gives lots of information about half lives
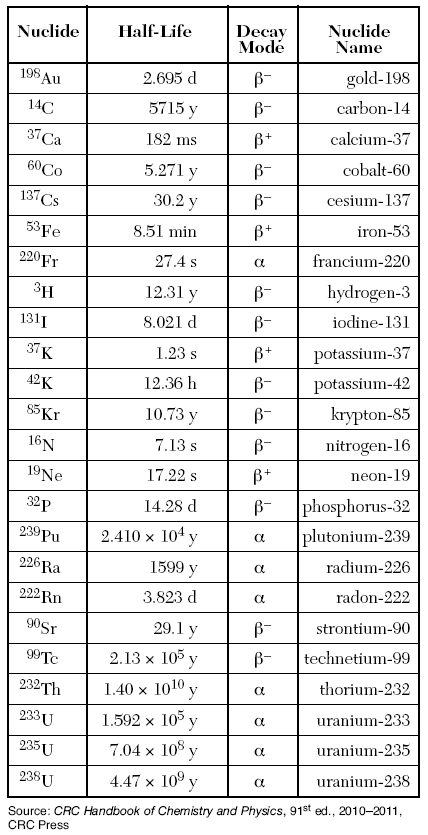
The time it takes for a certain isotope to go through a half-life is in the “half-Life” column
s= seconds
min = minutes
h = hours
d = dyas
y = years
Table D gives the selected units above.
The Decay Mode is what particle it relases when the radioisotope undergoes transmutation.
They will all be found on Table O.
Examples of the Math:
How many half lives does it take for 32 grams of Carbon-14 to decay into 2 grams?
To figure this out we can just keep dividing 24 and its quotients until we reach 2 grams.
32/2= 16 is one half-life, 16/2= 8 is two, 8/2= 4 is three, and 4/2= 2 is four half-lives.
It would take 4 half lives to reach 2 grams.
How long will it take for 24 grams of Gold-198 to decay into 3 grams?
First we can figure out how many half lives have to happen to reach 3 grams
Take 24g and divide it by 2 until you get 3g. It takes 3 times, so that is 3 half-lives
24/2= 12, 12/2= 6, 6/2= 3.
Then multiply the amount of half-lifes by the time of one half-life (given by Table N)
3 x 2.695d = 8.085 days
After 15.813 years how much Cobalt-60 is left from a 28 gram sample?
Like before we need to figure out how many half lives have occurred, this time we divide the time given by the time on Table N
15.813/5.271= 3 half lives
Now we can just divide 28 by 2, three times to get how much is left
28/2 = 14, 14/2= 7, and 7/2= 3.5 grams left
Uses of Radioisotopes:
→ Radioisotopes can have many uses in the real world:
Carbon-14 for example if used for the dating of biological remains.
Chemical Tracers →When isotopes are used to follow the path of material (such as fertilizer) in some sort of system.
They trace the path by following the particles which fall off.
They could also have medical applications (mostly to do with cancer)
I-131 is used to detect thyroid cancer.
Co-60 is used on tumors
Tc-99 is used to detect other types of cancer
Typically radioisotopes with longer half-lives are used for things like the dating of rocks or other remains, while those with shorter half-lives are used for medical purposes
The longer half-lives help with the elopement still holding up for a long time allowing us to see things from a long time ago.
The shorter half-lives allow for quick delivery or detection in medicine.
Unit 11- Nuclear Chemistry
DO NOT CLICK FLASHCARDS FROM HERE (OR STUDY) Click Here
* Tables N, and O, as well as the Periodic Table of Elements from the NYS Chemistry Reference table are very relevant to this unit.
*This is the last Unit for TOW chemistry
The Nucleus and Transmutation:
*Nuclear chemistry deals with Nuclei, and how they decay once they get too big and unstable.
Unstable Nuclei →they decay and emit particles due to the fact that they are so large. They contain lots of Protons and Neutrons, making them very heavy.
Lots of Energy is used in the Nucleus to keep all of the protons together, since they have the same charge and really don’t like to be shoved in the same nucleus.
The more protons there are the more unstable the Nucleus is.
Nuclei just mean more than one Nucleus.
When a Nucleus is unstable it will decay in a process known as Transmutation.
Transmutation → When the Nucleus of an Atom changes, changing the element.
When this happen the atom will release a radioactive particle, and the leftover Nucleus will become a different atom due to a different number of protons.
Energy is also released, as less energy is needed to hold less protons together.
The particles which can be released are shown on Table O.
The Number at the top left of the symbol is the mass (Protons + Neutrons)
The Mass of an alpha particle/ Helium atom is 4.
The Number below that one is the number of protons/ charge
The number of protons in an alpha particle is 2.
Penetration Power →The ability for a particle to pass through matter
Gamma radiation has the greatest penetrating power, since it has no mass or charge making it easier to pass through matter
Often in reaction gamma is omitted because it’s just energy, and dosent take much from the Nucleus.
Transmutation Reactions:
* These reactions show how Transmutation occurs, but there are two types (Artificial and Natural)
Natural Transmutation →When an Unstable Nuclei decays on it’s own in nature. Usually will happen with the half-life.
An equation will have one reactant (left side), and 2 products (right side)
Artificial Transmutation →When humans shoot a particle at an unstable nuclei causing it to break apart. picture it as like a laser zapping off a piece of the nucleus.
An equation will have 2 reactants and 2 products.
For the Equations the mass must be equal, and the number of protons total must be equal on both sides.
If in a natural Transmutation reaction, the reactant has a mass of 20, and has 7 protons, than the sum of the mass on the products must equal 20, and the sum of the protons must also equal 7.
Same with the sums of an artificial Transmutation reaction.
Examples:
Artificial Transmutation:
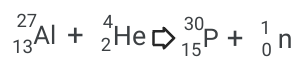
The sum of the top numbers(mass) is the same on both sides (31)
The sum of the bottom numbers (Protons) is also the same on both sides (15)
Aluminum is being bombarded with Helium (which can also be called an alpha particle.)
This releases a neutron when it hits the nucleus.
Natural Transmutation:
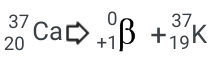
The sum of the mass for the products equals 27, and the sum of the protons equals 20
Ca is naturally decaying, and releases a positron, and made Potassium with the leftover Protons and neutrons.
Fission and Fusion:
Fusion → two lighter Nuclei combine to form one heavier nucleus with a release of energy.
What the sun does
Will usually involve Hydrogen and Helium
Two light atoms like H or He, will combine to form either a H or He with a heavier mass/ nucleus
Fission → Forcing a radioactive particle to collide with a large radioactive element, causing it to split apart and release lots of stored energy
Artificial Transmutation
What is done at Nuclear power plants.
One atom is split into TWO new elements rather than one.
Half-Lives:
→ The time it takes for a radioactive isotope to lose half of its mass.
It loses the mass due to natural transmuation and decay.
Table N, gives lots of information about half lives

The time it takes for a certain isotope to go through a half-life is in the “half-Life” column
s= seconds
min = minutes
h = hours
d = dyas
y = years
Table D gives the selected units above.
The Decay Mode is what particle it relases when the radioisotope undergoes transmutation.
They will all be found on Table O.
Examples of the Math:
How many half lives does it take for 32 grams of Carbon-14 to decay into 2 grams?
To figure this out we can just keep dividing 24 and its quotients until we reach 2 grams.
32/2= 16 is one half-life, 16/2= 8 is two, 8/2= 4 is three, and 4/2= 2 is four half-lives.
It would take 4 half lives to reach 2 grams.
How long will it take for 24 grams of Gold-198 to decay into 3 grams?
First we can figure out how many half lives have to happen to reach 3 grams
Take 24g and divide it by 2 until you get 3g. It takes 3 times, so that is 3 half-lives
24/2= 12, 12/2= 6, 6/2= 3.
Then multiply the amount of half-lifes by the time of one half-life (given by Table N)
3 x 2.695d = 8.085 days
After 15.813 years how much Cobalt-60 is left from a 28 gram sample?
Like before we need to figure out how many half lives have occurred, this time we divide the time given by the time on Table N
15.813/5.271= 3 half lives
Now we can just divide 28 by 2, three times to get how much is left
28/2 = 14, 14/2= 7, and 7/2= 3.5 grams left
Uses of Radioisotopes:
→ Radioisotopes can have many uses in the real world:
Carbon-14 for example if used for the dating of biological remains.
Chemical Tracers →When isotopes are used to follow the path of material (such as fertilizer) in some sort of system.
They trace the path by following the particles which fall off.
They could also have medical applications (mostly to do with cancer)
I-131 is used to detect thyroid cancer.
Co-60 is used on tumors
Tc-99 is used to detect other types of cancer
Typically radioisotopes with longer half-lives are used for things like the dating of rocks or other remains, while those with shorter half-lives are used for medical purposes
The longer half-lives help with the elopement still holding up for a long time allowing us to see things from a long time ago.
The shorter half-lives allow for quick delivery or detection in medicine.
 Knowt
Knowt