
Chapter 3: The Organic Molecules of Life
3.1 Organic Molecules
Chemistry can be divided into two major categories, those being the following:
Organic chemistry
Contains atoms of carbon and hydrogen.
Makeup portions of cells, tissues, and organs.
Inorganic chemistry
Does not contain a combination of carbon and hydrogen.
The Carbon Atom
A microscopic bacterial cell can contain thousands of different organic molecules.
In order for carbon to acquire four electrons to complete its outer shell, a carbon atom almost always shares electrons, and typically with the elements hydrogen, nitrogen, and oxygen—the elements that make up most of the weight of living organisms**.**
Because carbon is small and needs to acquire four electrons, carbon can bond with as many as four other elements.
Carbon atoms most often share electrons with other carbon atoms.
Hydrocarbons
Chains of carbon atoms that are also bonded only to hydrogen atoms.
Any carbon atom of a hydrocarbon molecule can start a branch chain, and a hydrocarbon can turn back on itself to form a ring compound.
Carbon can form double bonds with other atoms, including other carbon atoms.
The versatile nature of carbon means that it can form a variety of molecules with the same chemical formula but different structures.
Isomers
Molecules with different structures but the same combinations of atoms.
The chemistry of carbon leads to the substantial structural diversity of organic molecules. Since structure dictates function, this structural diversity means that these molecules have diverse roles.
The Carbon Skeleton and Functional Groups
Skeleton
Also known as the “backbone,” it is the carbon chain of an organic molecule.
Just as your skeleton accounts for your shape, so does the carbon skeleton of an organic molecule account for its shape.
The reactivity of an organic molecule is dependent mainly on the attached functional groups.
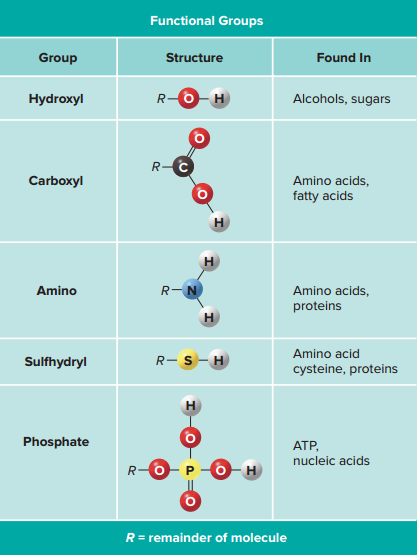
Functional group
A specific combination of bonded atoms that always has the same chemical properties and, therefore, always reacts in the same way, regardless of the particular carbon skeleton to which it is attached.
The functional groups of an organic molecule help determine its chemical properties.
Example: organic molecules, such as fats and proteins, containing carboxyl groups (⏤COOH) are both polar (hydrophilic) and weakly acidic.
Phosphate groups contribute to the structure of nucleic acids, such as DNA.
Proteins and amino acids possess the nitrogen-containing amino functional group (⏤NH2).
Because cells are composed mainly of water, the ability to interact with and be soluble in water profoundly affects the activity of organic molecules in cells.
Example: hydrocarbons are predominantly hydrophobic, but if a number of —OH functional groups are added, the molecule may be hydrophilic.
Functional groups also identify the types of reactions that the molecule will undergo.
Example: fats are formed by the interaction of molecules containing alcohols and carboxyl groups, and proteins are formed when the amino and carboxyl functional groups of nearby amino acids are linked.
3.2 The Biological Molecules of Cells
Biological molecules are grouped into only four categories, those being the following:
Carbohydrates
Bread
Potato
Corn
Rice
Pasta
Lipids
Cheese
Ice cream
Oil
Butter
Lard
Proteins
Milk
Meat
Tofu
Eggs
Nuts
Beans
Nucleic acids.
When you consume these biological molecules, your body breaks them down into smaller molecules or subunits; Your body then takes these subunits and builds from them the large macromolecules that make up your cells.
Foods contain nucleic acids, the type of biological molecule that forms the genetic material of all living organisms.
Monomers
Building blocks that form many of the biological molecules.
Polymers
The product of when multiple monomers join together.
A protein can contain hundreds of amino acid monomers, and a nucleic acid can contain hundreds of nucleotide monomers.
A polymer gets longer as monomers bond to one another.
Dehydration synthesis reaction
The most common type of chemical reaction that is used to build a polymer from a group of monomers.
It is called this because the equivalent of a water (H2O) molecule, meaning both an ⏤OH (hydroxyl group) and an H (hydrogen atom), is removed as the reaction occurs.
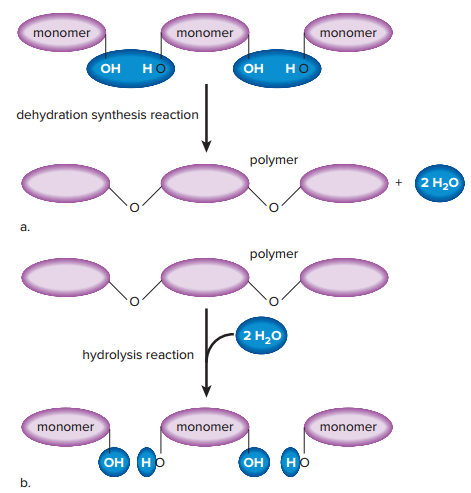
Hydrolysis reaction
The opposite of a dehydration synthesis reaction is used to break down a biological molecule.
During the reaction, an ⏤OH group from water attaches to one monomer, and an H from water attaches to the other monomer (water is used to break the bond holding monomers together).
Carbohydrates
In living organisms, carbohydrates are almost universally used as an immediate energy source.
Carbohydrates may exist either as saccharide (sugar) monomers or as polymers of saccharides.
Sugar glucose is a common monomer of carbohydrate polymers.
The term carbohydrate may refer to a single sugar molecule (monosaccharide), two bonded sugar molecules (disaccharide), or many sugar molecules bonded together (polysaccharide).
Monosaccharides: Energy Molecules
Monosaccharides are known as simple sugars because they have only a single sugar molecule.
A simple sugar can have a carbon backbone consisting of three to seven carbons.
Monosaccharides, and carbohydrates in general, often possess many polar —OH functional groups, which make them soluble in water.
In a water environment, such as that within our cells, carbohydrates often form a ringlike structure.
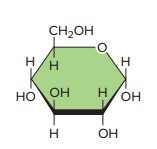
Glucose, with six carbon atoms, has a molecular formula of C6,H12,O6.
Glucose has two important isomers, those being the following:
Fructose
Galactose,
Photosynthetic organisms, such as plants and bacteria, manufacture glucose using energy from the sun; This glucose is used as the preferred immediate source of energy for nearly all types of organisms.
Ribose and deoxyribose, with five carbon atoms, are significant because they are found in the nucleic acids RNA and DNA.
Disaccharides: Varied Uses
A disaccharide contains two monosaccharides linked together by a dehydration synthesis reaction.
Some common disaccharides are maltose, sucrose, and lactose.
Maltose is a disaccharide that contains two glucose subunits.
Our bodies digest sucrose into its two monomers, glucose and fructose, and later, the fructose is changed to glucose, our usual energy source.
If the body doesn’t need more energy at the moment, the glucose can be metabolized to fat.
Fat is the body’s primary energy storage form.
Polysaccharides as Energy Storage Molecules
Polysaccharides
Polymers of monosaccharides, usually glucose.
Polysaccharides cannot easily pass through the plasma membrane and are kept (stored) within the cell.
Some types of polysaccharides function as short-term energy storage molecules because they are much larger than a monosaccharide and are relatively insoluble.
Plants store glucose as starch.
Animals store glucose as glycogen, which is more highly branched than starch.
Branching subjects a polysaccharide to more attacks by hydrolytic enzymes; therefore, branching makes a polysaccharide easier to break down.
Hormones, such as insulin, control the storage and release of glucose from liver cells.
Polysaccharides as Structural Molecules
Some types of polysaccharides function as structural components of cells
Example: cellulose, which is the most abundant of all carbohydrates.
Plant and algal cell walls all contain cellulose, and therefore it may be found in all of the tissues of a plant.
The bonds joining the glucose subunits in cellulose are different from those found in starch and glycogen; As a result, the molecule does not spiral or have branches.
The long glucose chains are held parallel to each other by hydrogen bonding to form strong microfibrils, which are grouped into fibers.
The fibers crisscross within plant cell walls for even more strength.
The different bond structure means that the digestive systems of animals can’t hydrolyze cellulose, but some microorganisms have this ability.
Cows and other ruminants have an internal pouch where microorganisms break down cellulose to glucose; In humans, cellulose has the benefit of serving as dietary fiber, which maintains regular elimination and digestive system health.
Chitin
A polymer of glucose molecules
In chitin, each glucose subunit has an amino group (⏤NH2 ) attached to it.
Chitin is found in a variety of organisms, including animals and fungi.
In animals such as insects, crabs, and lobsters, chitin is found in the external skeleton or exoskeleton.
Even though chitin, like cellulose, is not digestible by humans, it still has many good uses.
Seeds are coated with chitin, and this protects them from attack by soil fungi.
Because chitin also has antibacterial and antiviral properties, it is processed and used in medicine as a wound dressing and suture material.
Chitin is also useful during the production of cosmetics and various foods.
Lipids
Molecules classified as lipids are varied; however, they all share the characteristic of being hydrophobic and insoluble in water.
Lipids possess long, nonpolar hydrocarbon chains and a relative lack of hydrophilic functional groups.
In general, the difference between fats and oils is that fats are solid at room temperature, while oils are liquid at room temperature.
In animals, fats are used for both insulation and long-term energy storage.
Instead of fats, plants use oils for long-term energy storage.
In animals, the secretions of oil glands help waterproof skin, hair, and feathers.
Fats and Oils: Long-term Energy Storage
Fats and oils contain two types of subunit molecules, those being the following:
Glycerol
Contains three ⏤OH groups; The ⏤OH groups are polar; therefore, glycerol is soluble in water.
Fatty Acid
Has a long chain of carbon atoms bonded only to hydrogen, with a carboxyl group at one end; A fat or an oil forms when the carboxyl portions of three fatty acids react with the —OH groups of glycerol.
This is a dehydration synthesis reaction because, in addition to a fat molecule, three molecules of water result.
Fats and oils are degraded during a hydrolysis reaction, in which water is added to the molecule.
Fats and oils are degraded during a hydrolysis reaction, in which water is added to the molecule.
Triglycerides
Another name for fats and oils
It is logical that fats and oils are the body’s primary long-term energy storage molecules.
Fatty acids are the primary components of fats and oils.
Most of the fatty acids in cells contain 16 or 18 carbon atoms per molecule.
Fatty acids can come in two forms, those being the following:
Saturated
Have no double bonds between the carbon atoms.
Unsaturated
Have double bonds in the carbon chain wherever the number of hydrogens is less than two per carbon atom.
The carbon chain is saturated with all the hydrogens it can hold; saturation or unsaturation of a fatty acid determines its chemical and physical properties.
Oils are liquids at room temperature because they contain unsaturated fatty acids.
Things such as butter are solid at room temperature because it contains mostly saturated fatty acids.
The saturated fatty acid chains can pack together more tightly because they have no kinks.
Trans Fats
A name given to unsaturated fat if it contains a C==C bond with the hydrogen atoms located on opposite sides of the bond.
Trans fats are often formed during the processing of foods, such as margarine, baked goods, and fried foods, to make the product more solid.
The label “partially-hydrogenated oils” typically indicates the presence of trans fats in a food product.
Saturated fats and trans fats tend to stick together in the blood, forming plaque.
The accumulation of plaque in the blood vessels causes a disease called atherosclerosis; atherosclerosis contributes to high blood pressure and heart attacks.
Unsaturated oils, including monounsaturated and polyunsaturated
have been found to be protective against atherosclerosis because they do not stick together as much in the blood.
These healthy oils are found in abundance in olive oil, canola oil, and certain fish.
Phospholipids: Membrane Components
Phospholipids contain a phosphate functional group.
A phospholipid is constructed like a triglyceride, except that, in place of the third fatty acid attached to glycerol, there is a charged phosphate group.
The phosphate group is usually bonded to another polar functional group; Thus, one end of the molecule is hydrophilic and water soluble.
Because phospholipids have both hydrophilic (polar) heads and hydrophobic (nonpolar) tails, they tend to arrange themselves so that only the polar heads interact with the watery environment outside, and the nonpolar tails crowd inward away from the water.
Between two compartments of water, such as the outside and inside of a cell, phospholipids become a bilayer in which the polar heads project outward, and the nonpolar tails project inward.
The bulk of the plasma membrane that surrounds cells consists of a fairly fluid phospholipid bilayer, as do all the other membranes in the cell; a plasma membrane is essential to the structure and function of a cell, and thus phospholipids are vital to humans, and other organisms.
Steroids: Four Fused Rings
Steroids
Lipids that possess a unique carbon skeleton made of four fused rings.
Highly diverse
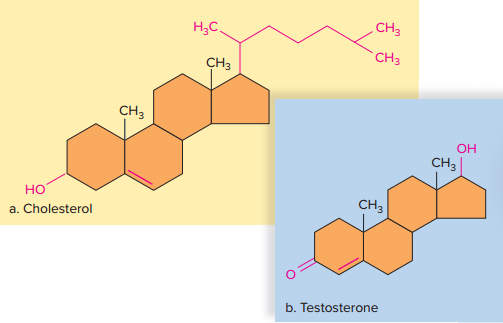
Unlike other lipids, steroids do not contain fatty acids, but they are similar to other lipids because they are insoluble in water.
The types of steroids differ primarily in the types of functional groups attached to their carbon skeleton.
Cholesterol
A component of an animal cell’s plasma membrane, it is the precursor of other steroids, such as the sex hormones testosterone and estrogen.
The male sex hormone, testosterone, is formed primarily in the testes, and the female sex hormone, estrogen, is formed primarily in the ovaries.
Testosterone and estrogen differ only in the functional groups attached to the same carbon skeleton, yet, they have a profound effect on the bodies and the sexuality of humans and other animals.
Anabolic steroids, such as synthetic testosterone, can be used to increase muscle mass.
The result is usually unfortunate as The presence of the steroid in the body upsets the normal hormonal balance: The testes atrophy (shrink and weaken), and males may develop breasts; females tend to grow facial hair and lose hair on their heads.
Because steroid use gives athletes an unfair advantage and destroys their health—heart, kidney, liver, and psychological disorders are common—professional athletic associations ban anabolic steroids.
Proteins
Proteins are of primary importance in the structure and function of cells; examples of what they are involved in include the following:
Support
Metabolism
Transport
Defense
Regulation
Amino Acids: Monomers of Proteins
Proteins are polymers, and their monomers are called amino acids.
Amino acids have a unique carbon skeleton in which a central carbon atom bonds to a hydrogen atom, two functional groups, and a variable side chain or R group.
In amino acids, one of the two functional groups is an ⏤NH2 (amino group), and the other is a ⏤COOH (an acid, or carboxyl, group).
There are 20 different amino acids, which differ by their particular R group.
The R groups range in complexity from a single hydrogen atom to a complicated ring structure.
The unique chemical properties of an amino acid depends on the chemical properties of the R group.
Peptides
A peptide is formed when two amino acids are joined by a dehydration synthesis reaction between the carboxyl group of one and the amino group of another.
The resulting covalent bond between two amino acids is called a peptide bond.
The atoms associated with the peptide bond share the electrons unevenly because oxygen is more electronegative than nitrogen.
Therefore, the peptide bond is polar, and hydrogen bonding is possible between the C==O of one amino acid and the N⏤H of another amino acid in a polypeptide.
Polypeptide
A chain of many amino acids joined by peptide bonds.
Proteins are polypeptide chains that have folded into complex shapes; a protein may contain one or more polypeptide chains. While some proteins are small, others are composed of a very large number of amino acids.
Ribonuclease, a small protein that breaks down RNA, contains barely more than 100 amino acids.
Some proteins are very large, such as titin, which contains over 33,000 amino acids.
Titin
An integral part of the structure of your muscles.
Without titin, your muscles would not function properly.
The amino acid sequence determines a protein’s final three-dimensional shape and, thus, its function.
Each polypeptide has its own typical sequence.
Proteins that have an abnormal sequence of amino acids often have the wrong shape and cannot function properly.
Shape of Proteins
Primary, Secondary, Tertiary, and Quaternary structures.
The name is given to the multiple levels of a protein’s structure.
A protein’s sequence of amino acids is called its primary structure.
The secondary structure of a protein results when portions of the amino acid chain take on a specific orientation in space, depending on the number and identity of the amino acids present in the chain.
Hydrogen bonds between nearby peptide bonds maintain the secondary structure of a protein.
Any polypeptide may contain one or more secondary structure regions within the same chain.
The tertiary structure of a protein is its overall three-dimensional shape that results from the folding and twisting of its secondary structure.
The tertiary structure is held in place by interactions between the R groups of amino acids making up the helices and beta-pleated sheets within the polypeptide.
The tertiary structure of a protein determines its function, and this structure can be affected by environmental conditions, such as pH and temperature.
Denatured
Broken down and inactivated.
Some proteins, such as hemoglobin and insulin, have a quaternary structure because they contain more than one polypeptide chain.
Each polypeptide chain in such a protein has its own primary, secondary, and tertiary structures. The quaternary structure is determined by how the individual polypeptide chains interact.
Nucleic Acids
Deoxyribonucleic acid
DNA
Acts as the location within the cell where the genetic information is stored.
Ribonucleic acid
RNA
The molecule that aids in transcribing and translating DNA into proteins.
Both DNA and RNA are the nucleic acids found in cells.
Each DNA molecule contains many genes, which specify the sequence of the amino acids in proteins.
Nucleic acids are polymers in which the monomer is called a nucleotide.
All nucleotides, whether they are in DNA or RNA, have three parts, those being the following:
A phosphate (a —PO4 functional group)
A 5-carbon sugar and a nitrogen-containing base
Deoxyribose has one less oxygen than ribose.
Each nucleotide of DNA contains one of four nitrogen-containing bases:
Adenine (A)
Guanine (G)
Cytosine (C)
Thymine (T)
DNA is a double helix, meaning that two strands spiral around one another.
In each strand, the backbone of the molecule is composed of phosphates bonded to sugars, and the bases project to the inside.
The base guanine (G) is always paired with cytosine (C), and the base adenine (A) is always paired with thymine (T).
This is called complementary base pairing.
Complementary base pairing holds the two strands together and is very important when DNA makes a copy of itself.
Replication
When DNA makes a copy of itself.
RNA differs from DNA not only by its sugar but also because it uses the base uracil (U) instead of thymine. Whereas DNA is double-stranded, RNA is single-stranded.
Complementary base pairing also allows DNA to pass genetic information to RNA.
The information is stored in the sequence of bases.
DNA has a triplet code called codons, and every three bases stand for one of the 20 amino acids in cells.
Once you know the sequence of bases in a gene, you know the sequence of amino acids in a polypeptide.
As a result of the Human Genome Project, we now have a complete sequence of each of the 3.2 billion bases and roughly 23,000 genes in humans.
ATP: An Energy Molecule
In addition to being one of the subunits of nucleic acids, the nucleotide adenine has a derivative with a metabolic function that is very important to most cells.
When the addition of three phosphate groups modifies adenosine (adenine plus ribose), it becomes adenosine triphosphate (ATP)
ATP acts as an energy carrier in cells.
Chapter 3: The Organic Molecules of Life
3.1 Organic Molecules
Chemistry can be divided into two major categories, those being the following:
Organic chemistry
Contains atoms of carbon and hydrogen.
Makeup portions of cells, tissues, and organs.
Inorganic chemistry
Does not contain a combination of carbon and hydrogen.
The Carbon Atom
A microscopic bacterial cell can contain thousands of different organic molecules.
In order for carbon to acquire four electrons to complete its outer shell, a carbon atom almost always shares electrons, and typically with the elements hydrogen, nitrogen, and oxygen—the elements that make up most of the weight of living organisms**.**
Because carbon is small and needs to acquire four electrons, carbon can bond with as many as four other elements.
Carbon atoms most often share electrons with other carbon atoms.
Hydrocarbons
Chains of carbon atoms that are also bonded only to hydrogen atoms.
Any carbon atom of a hydrocarbon molecule can start a branch chain, and a hydrocarbon can turn back on itself to form a ring compound.
Carbon can form double bonds with other atoms, including other carbon atoms.
The versatile nature of carbon means that it can form a variety of molecules with the same chemical formula but different structures.
Isomers
Molecules with different structures but the same combinations of atoms.
The chemistry of carbon leads to the substantial structural diversity of organic molecules. Since structure dictates function, this structural diversity means that these molecules have diverse roles.
The Carbon Skeleton and Functional Groups
Skeleton
Also known as the “backbone,” it is the carbon chain of an organic molecule.
Just as your skeleton accounts for your shape, so does the carbon skeleton of an organic molecule account for its shape.
The reactivity of an organic molecule is dependent mainly on the attached functional groups.

Functional group
A specific combination of bonded atoms that always has the same chemical properties and, therefore, always reacts in the same way, regardless of the particular carbon skeleton to which it is attached.
The functional groups of an organic molecule help determine its chemical properties.
Example: organic molecules, such as fats and proteins, containing carboxyl groups (⏤COOH) are both polar (hydrophilic) and weakly acidic.
Phosphate groups contribute to the structure of nucleic acids, such as DNA.
Proteins and amino acids possess the nitrogen-containing amino functional group (⏤NH2).
Because cells are composed mainly of water, the ability to interact with and be soluble in water profoundly affects the activity of organic molecules in cells.
Example: hydrocarbons are predominantly hydrophobic, but if a number of —OH functional groups are added, the molecule may be hydrophilic.
Functional groups also identify the types of reactions that the molecule will undergo.
Example: fats are formed by the interaction of molecules containing alcohols and carboxyl groups, and proteins are formed when the amino and carboxyl functional groups of nearby amino acids are linked.
3.2 The Biological Molecules of Cells
Biological molecules are grouped into only four categories, those being the following:
Carbohydrates
Bread
Potato
Corn
Rice
Pasta
Lipids
Cheese
Ice cream
Oil
Butter
Lard
Proteins
Milk
Meat
Tofu
Eggs
Nuts
Beans
Nucleic acids.
When you consume these biological molecules, your body breaks them down into smaller molecules or subunits; Your body then takes these subunits and builds from them the large macromolecules that make up your cells.
Foods contain nucleic acids, the type of biological molecule that forms the genetic material of all living organisms.
Monomers
Building blocks that form many of the biological molecules.
Polymers
The product of when multiple monomers join together.
A protein can contain hundreds of amino acid monomers, and a nucleic acid can contain hundreds of nucleotide monomers.
A polymer gets longer as monomers bond to one another.
Dehydration synthesis reaction
The most common type of chemical reaction that is used to build a polymer from a group of monomers.
It is called this because the equivalent of a water (H2O) molecule, meaning both an ⏤OH (hydroxyl group) and an H (hydrogen atom), is removed as the reaction occurs.

Hydrolysis reaction
The opposite of a dehydration synthesis reaction is used to break down a biological molecule.
During the reaction, an ⏤OH group from water attaches to one monomer, and an H from water attaches to the other monomer (water is used to break the bond holding monomers together).
Carbohydrates
In living organisms, carbohydrates are almost universally used as an immediate energy source.
Carbohydrates may exist either as saccharide (sugar) monomers or as polymers of saccharides.
Sugar glucose is a common monomer of carbohydrate polymers.
The term carbohydrate may refer to a single sugar molecule (monosaccharide), two bonded sugar molecules (disaccharide), or many sugar molecules bonded together (polysaccharide).
Monosaccharides: Energy Molecules
Monosaccharides are known as simple sugars because they have only a single sugar molecule.
A simple sugar can have a carbon backbone consisting of three to seven carbons.
Monosaccharides, and carbohydrates in general, often possess many polar —OH functional groups, which make them soluble in water.
In a water environment, such as that within our cells, carbohydrates often form a ringlike structure.

Glucose, with six carbon atoms, has a molecular formula of C6,H12,O6.
Glucose has two important isomers, those being the following:
Fructose
Galactose,
Photosynthetic organisms, such as plants and bacteria, manufacture glucose using energy from the sun; This glucose is used as the preferred immediate source of energy for nearly all types of organisms.
Ribose and deoxyribose, with five carbon atoms, are significant because they are found in the nucleic acids RNA and DNA.
Disaccharides: Varied Uses
A disaccharide contains two monosaccharides linked together by a dehydration synthesis reaction.
Some common disaccharides are maltose, sucrose, and lactose.
Maltose is a disaccharide that contains two glucose subunits.
Our bodies digest sucrose into its two monomers, glucose and fructose, and later, the fructose is changed to glucose, our usual energy source.
If the body doesn’t need more energy at the moment, the glucose can be metabolized to fat.
Fat is the body’s primary energy storage form.
Polysaccharides as Energy Storage Molecules
Polysaccharides
Polymers of monosaccharides, usually glucose.
Polysaccharides cannot easily pass through the plasma membrane and are kept (stored) within the cell.
Some types of polysaccharides function as short-term energy storage molecules because they are much larger than a monosaccharide and are relatively insoluble.
Plants store glucose as starch.
Animals store glucose as glycogen, which is more highly branched than starch.
Branching subjects a polysaccharide to more attacks by hydrolytic enzymes; therefore, branching makes a polysaccharide easier to break down.
Hormones, such as insulin, control the storage and release of glucose from liver cells.
Polysaccharides as Structural Molecules
Some types of polysaccharides function as structural components of cells
Example: cellulose, which is the most abundant of all carbohydrates.
Plant and algal cell walls all contain cellulose, and therefore it may be found in all of the tissues of a plant.
The bonds joining the glucose subunits in cellulose are different from those found in starch and glycogen; As a result, the molecule does not spiral or have branches.
The long glucose chains are held parallel to each other by hydrogen bonding to form strong microfibrils, which are grouped into fibers.
The fibers crisscross within plant cell walls for even more strength.
The different bond structure means that the digestive systems of animals can’t hydrolyze cellulose, but some microorganisms have this ability.
Cows and other ruminants have an internal pouch where microorganisms break down cellulose to glucose; In humans, cellulose has the benefit of serving as dietary fiber, which maintains regular elimination and digestive system health.
Chitin
A polymer of glucose molecules
In chitin, each glucose subunit has an amino group (⏤NH2 ) attached to it.
Chitin is found in a variety of organisms, including animals and fungi.
In animals such as insects, crabs, and lobsters, chitin is found in the external skeleton or exoskeleton.
Even though chitin, like cellulose, is not digestible by humans, it still has many good uses.
Seeds are coated with chitin, and this protects them from attack by soil fungi.
Because chitin also has antibacterial and antiviral properties, it is processed and used in medicine as a wound dressing and suture material.
Chitin is also useful during the production of cosmetics and various foods.
Lipids
Molecules classified as lipids are varied; however, they all share the characteristic of being hydrophobic and insoluble in water.
Lipids possess long, nonpolar hydrocarbon chains and a relative lack of hydrophilic functional groups.
In general, the difference between fats and oils is that fats are solid at room temperature, while oils are liquid at room temperature.
In animals, fats are used for both insulation and long-term energy storage.
Instead of fats, plants use oils for long-term energy storage.
In animals, the secretions of oil glands help waterproof skin, hair, and feathers.
Fats and Oils: Long-term Energy Storage
Fats and oils contain two types of subunit molecules, those being the following:
Glycerol
Contains three ⏤OH groups; The ⏤OH groups are polar; therefore, glycerol is soluble in water.
Fatty Acid
Has a long chain of carbon atoms bonded only to hydrogen, with a carboxyl group at one end; A fat or an oil forms when the carboxyl portions of three fatty acids react with the —OH groups of glycerol.
This is a dehydration synthesis reaction because, in addition to a fat molecule, three molecules of water result.
Fats and oils are degraded during a hydrolysis reaction, in which water is added to the molecule.
Fats and oils are degraded during a hydrolysis reaction, in which water is added to the molecule.
Triglycerides
Another name for fats and oils
It is logical that fats and oils are the body’s primary long-term energy storage molecules.
Fatty acids are the primary components of fats and oils.
Most of the fatty acids in cells contain 16 or 18 carbon atoms per molecule.
Fatty acids can come in two forms, those being the following:
Saturated
Have no double bonds between the carbon atoms.
Unsaturated
Have double bonds in the carbon chain wherever the number of hydrogens is less than two per carbon atom.
The carbon chain is saturated with all the hydrogens it can hold; saturation or unsaturation of a fatty acid determines its chemical and physical properties.
Oils are liquids at room temperature because they contain unsaturated fatty acids.
Things such as butter are solid at room temperature because it contains mostly saturated fatty acids.
The saturated fatty acid chains can pack together more tightly because they have no kinks.
Trans Fats
A name given to unsaturated fat if it contains a C==C bond with the hydrogen atoms located on opposite sides of the bond.
Trans fats are often formed during the processing of foods, such as margarine, baked goods, and fried foods, to make the product more solid.
The label “partially-hydrogenated oils” typically indicates the presence of trans fats in a food product.
Saturated fats and trans fats tend to stick together in the blood, forming plaque.
The accumulation of plaque in the blood vessels causes a disease called atherosclerosis; atherosclerosis contributes to high blood pressure and heart attacks.
Unsaturated oils, including monounsaturated and polyunsaturated
have been found to be protective against atherosclerosis because they do not stick together as much in the blood.
These healthy oils are found in abundance in olive oil, canola oil, and certain fish.
Phospholipids: Membrane Components
Phospholipids contain a phosphate functional group.
A phospholipid is constructed like a triglyceride, except that, in place of the third fatty acid attached to glycerol, there is a charged phosphate group.
The phosphate group is usually bonded to another polar functional group; Thus, one end of the molecule is hydrophilic and water soluble.
Because phospholipids have both hydrophilic (polar) heads and hydrophobic (nonpolar) tails, they tend to arrange themselves so that only the polar heads interact with the watery environment outside, and the nonpolar tails crowd inward away from the water.
Between two compartments of water, such as the outside and inside of a cell, phospholipids become a bilayer in which the polar heads project outward, and the nonpolar tails project inward.
The bulk of the plasma membrane that surrounds cells consists of a fairly fluid phospholipid bilayer, as do all the other membranes in the cell; a plasma membrane is essential to the structure and function of a cell, and thus phospholipids are vital to humans, and other organisms.
Steroids: Four Fused Rings
Steroids
Lipids that possess a unique carbon skeleton made of four fused rings.
Highly diverse

Unlike other lipids, steroids do not contain fatty acids, but they are similar to other lipids because they are insoluble in water.
The types of steroids differ primarily in the types of functional groups attached to their carbon skeleton.
Cholesterol
A component of an animal cell’s plasma membrane, it is the precursor of other steroids, such as the sex hormones testosterone and estrogen.
The male sex hormone, testosterone, is formed primarily in the testes, and the female sex hormone, estrogen, is formed primarily in the ovaries.
Testosterone and estrogen differ only in the functional groups attached to the same carbon skeleton, yet, they have a profound effect on the bodies and the sexuality of humans and other animals.
Anabolic steroids, such as synthetic testosterone, can be used to increase muscle mass.
The result is usually unfortunate as The presence of the steroid in the body upsets the normal hormonal balance: The testes atrophy (shrink and weaken), and males may develop breasts; females tend to grow facial hair and lose hair on their heads.
Because steroid use gives athletes an unfair advantage and destroys their health—heart, kidney, liver, and psychological disorders are common—professional athletic associations ban anabolic steroids.
Proteins
Proteins are of primary importance in the structure and function of cells; examples of what they are involved in include the following:
Support
Metabolism
Transport
Defense
Regulation
Amino Acids: Monomers of Proteins
Proteins are polymers, and their monomers are called amino acids.
Amino acids have a unique carbon skeleton in which a central carbon atom bonds to a hydrogen atom, two functional groups, and a variable side chain or R group.
In amino acids, one of the two functional groups is an ⏤NH2 (amino group), and the other is a ⏤COOH (an acid, or carboxyl, group).
There are 20 different amino acids, which differ by their particular R group.
The R groups range in complexity from a single hydrogen atom to a complicated ring structure.
The unique chemical properties of an amino acid depends on the chemical properties of the R group.
Peptides
A peptide is formed when two amino acids are joined by a dehydration synthesis reaction between the carboxyl group of one and the amino group of another.
The resulting covalent bond between two amino acids is called a peptide bond.
The atoms associated with the peptide bond share the electrons unevenly because oxygen is more electronegative than nitrogen.
Therefore, the peptide bond is polar, and hydrogen bonding is possible between the C==O of one amino acid and the N⏤H of another amino acid in a polypeptide.
Polypeptide
A chain of many amino acids joined by peptide bonds.
Proteins are polypeptide chains that have folded into complex shapes; a protein may contain one or more polypeptide chains. While some proteins are small, others are composed of a very large number of amino acids.
Ribonuclease, a small protein that breaks down RNA, contains barely more than 100 amino acids.
Some proteins are very large, such as titin, which contains over 33,000 amino acids.
Titin
An integral part of the structure of your muscles.
Without titin, your muscles would not function properly.
The amino acid sequence determines a protein’s final three-dimensional shape and, thus, its function.
Each polypeptide has its own typical sequence.
Proteins that have an abnormal sequence of amino acids often have the wrong shape and cannot function properly.
Shape of Proteins
Primary, Secondary, Tertiary, and Quaternary structures.
The name is given to the multiple levels of a protein’s structure.
A protein’s sequence of amino acids is called its primary structure.
The secondary structure of a protein results when portions of the amino acid chain take on a specific orientation in space, depending on the number and identity of the amino acids present in the chain.
Hydrogen bonds between nearby peptide bonds maintain the secondary structure of a protein.
Any polypeptide may contain one or more secondary structure regions within the same chain.
The tertiary structure of a protein is its overall three-dimensional shape that results from the folding and twisting of its secondary structure.
The tertiary structure is held in place by interactions between the R groups of amino acids making up the helices and beta-pleated sheets within the polypeptide.
The tertiary structure of a protein determines its function, and this structure can be affected by environmental conditions, such as pH and temperature.
Denatured
Broken down and inactivated.
Some proteins, such as hemoglobin and insulin, have a quaternary structure because they contain more than one polypeptide chain.
Each polypeptide chain in such a protein has its own primary, secondary, and tertiary structures. The quaternary structure is determined by how the individual polypeptide chains interact.
Nucleic Acids
Deoxyribonucleic acid
DNA
Acts as the location within the cell where the genetic information is stored.
Ribonucleic acid
RNA
The molecule that aids in transcribing and translating DNA into proteins.
Both DNA and RNA are the nucleic acids found in cells.
Each DNA molecule contains many genes, which specify the sequence of the amino acids in proteins.
Nucleic acids are polymers in which the monomer is called a nucleotide.
All nucleotides, whether they are in DNA or RNA, have three parts, those being the following:
A phosphate (a —PO4 functional group)
A 5-carbon sugar and a nitrogen-containing base
Deoxyribose has one less oxygen than ribose.
Each nucleotide of DNA contains one of four nitrogen-containing bases:
Adenine (A)
Guanine (G)
Cytosine (C)
Thymine (T)
DNA is a double helix, meaning that two strands spiral around one another.
In each strand, the backbone of the molecule is composed of phosphates bonded to sugars, and the bases project to the inside.
The base guanine (G) is always paired with cytosine (C), and the base adenine (A) is always paired with thymine (T).
This is called complementary base pairing.
Complementary base pairing holds the two strands together and is very important when DNA makes a copy of itself.
Replication
When DNA makes a copy of itself.
RNA differs from DNA not only by its sugar but also because it uses the base uracil (U) instead of thymine. Whereas DNA is double-stranded, RNA is single-stranded.
Complementary base pairing also allows DNA to pass genetic information to RNA.
The information is stored in the sequence of bases.
DNA has a triplet code called codons, and every three bases stand for one of the 20 amino acids in cells.
Once you know the sequence of bases in a gene, you know the sequence of amino acids in a polypeptide.
As a result of the Human Genome Project, we now have a complete sequence of each of the 3.2 billion bases and roughly 23,000 genes in humans.
ATP: An Energy Molecule
In addition to being one of the subunits of nucleic acids, the nucleotide adenine has a derivative with a metabolic function that is very important to most cells.
When the addition of three phosphate groups modifies adenosine (adenine plus ribose), it becomes adenosine triphosphate (ATP)
ATP acts as an energy carrier in cells.
 Knowt
Knowt