
Chapter 18 - Acid-Base
The proton is surrounded by water molecules, forming H-bonded species with the general formula (H2O)nH+. The charge density of the proton is so great that it attracts water molecules exceptionally strongly, covalently attaching to one of the lone electron pairs of a water molecule's O atom to form a water molecule.
H3O+ is formed as a hydronium ion, which makes H bonds with many additional water molecules. The formula written below shows an example of a formalized formalized formalized formalized formalized.
The hydrated proton is generally presented to illustrate the active function of water and the proton-water interaction [however, for convenience, we occasionally show the unhydrated proton].
Display it as H+(aq)] thus an Arrhenius acid may be defined as a chemical that produces H3O+ in water:
HA(aq) + H2O(l) ⟶ A– (aq) + H3O+(aq) − H + H A + + H O A O H H H
Typical Arrhenius acids include HCl, HNO3, and HCN, whereas typical Arrhenius bases include NaOH, KOH, and Ba(OH)2.
Arrhenius bases have distinct OH ions in their structures because they are ionic compounds, but Arrhenius acids never contain discrete H+ ions. Instead, they have covalently linked H atoms that ionize when acid molecules dissolve in water.
When an acid and a base react, they neutralize each other. As we will see, the definition of this word has evolved, but in the Arrhenius sense, neutralization happens when the H+ from the acid and the OH from the base combine to produce H2O.
Arrhenius was able to explain a fundamental aspect regarding neutralization: no matter which strong acid and a strong base react, and no matter which salt emerges, H°rxn equals 55.9 kJ per molecule.
A mole of water was produced. According to Arrhenius, the enthalpy change is always the same, identical because the reaction always produces the same product—a hydrogen ion and a hydroxide ion in water:
H+(aq) + OH−(aq) ⟶ H2O(l) ΔH°rxn = −55.9 kJ
The dissolved salt that is present, for example, NaCl in the reaction of sodium hydroxide with hydrochloric acid, Na+(aq) + OH−(aq) + H+(aq) + Cl−(aq) ⟶ Na+(aq) + Cl−(aq) + H2O(l) exists as hydrated spectator ions and does not affect ΔH°rxn.
Despite its relevance at the time, the Arrhenius definition quickly revealed its shortcomings. Arrhenius and others discovered that even though some compounds lack distinct OH ions, they nevertheless act as bases. In water, for example, NH3 and K2CO3 both produce OH.
To cover organisms that lack OH– ions in their structures, broader acid-base classifications are necessary. Water binds the proton produced by acid in an aqueous solution to generate a hydrated species denoted by H3O+ (aq).
Acids include H and yield H3O+ in water, bases contain OH and yield OH in water, and an acid-base reaction is the reaction of H+ and OH to generate H2O, according to the Arrhenius definition.
Many chemicals that produce OH ions in water do not include OH in their formulae, which is a significant restriction of the Arrhenius concept. Ammonia, amines, and numerous salts of weak acids, such as NaF, are examples.
Another constraint was that water had to be transported to function as a solvent in acid-base reactions. In the early twentieth century, J. N. Brnsted and others, T. M. Lowry proposed definitions that eliminate these constraints.
According to the Brasted-Lowry acid-base definition, an acid is any substance that provides an H+ ion as a proton donor. H must be present in the formula of an acid; HNO3 and H2PO4 are examples, as they are only two of countless instances. Arrhenius did everything. Brasted-Lowry acids are acids.
A base is any substance that takes an H+ ion as a proton acceptor. A foundation is required, for which they contain a single pair of electrons to bind H+; NH3, CO3 are a few examples.
2, as well as F, as well as the letter OH. Brasted-Lowry bases are not Arrhenius bases, but they are all bases. The Brasted-Lowry base OH is found in Arrhenius bases.
According to this viewpoint, an acid-base reaction happens when one species contributes a proton and another species takes it at the same time: an acid-base reaction is therefore a proton transfer event. Acid-base reactions can occur in gases, nonaqueous solutions, heterogeneous mixtures, and aqueous solutions.
An acid-base reaction happens even when no acid is present, according to this definition, (or a base) simply dissolves in water because water serves as a proton acceptor (or a base).
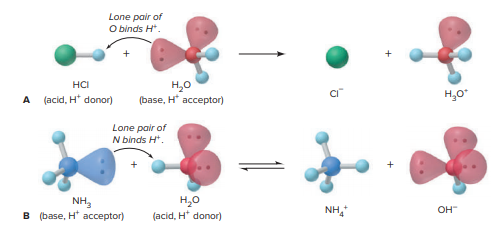
Water receives a proton from acid, as shown in the image attached below. Water receives a proton from acid (Figure 18.1A). When HCl dissolves in water, it transfers one H+ ion (a proton) from HCl to H2O, where it attaches to a lone pair of electrons on the O atom, creating H3O+. As a result, HCl (the acid) possesses H+ was provided, and H2O (the base) received it:
HCl(g) + H2O(l) Cl −(aq) + H3O+(aq)
A Brasted-Lowry acid-base reaction is the dissolution of an acid or a base in water. A The dissolving of the acid HCl in the base water. B, The acid water dissolves the base NH3.
A proton from water is accepted by the base (Figure 18.1B). When ammonia dissolves in water, and H+ from H2O is transferred to the lone pair of N, resulting in the formation of NH4 + H2O is converted into an OH ion:
NH3(aq) + H2O(l) NH4 +(aq) + OH−(aq)
In this example, H2O (the acid) gave the H+ and NH3 (the base) took it. H2O is amphiprotic, which means it may function as a base (accepts an H+) in one circumstance and an acid (donates an H+) in the other.
Many additional species are also amphiprotic
Chapter 18 - Acid-Base
The proton is surrounded by water molecules, forming H-bonded species with the general formula (H2O)nH+. The charge density of the proton is so great that it attracts water molecules exceptionally strongly, covalently attaching to one of the lone electron pairs of a water molecule's O atom to form a water molecule.
H3O+ is formed as a hydronium ion, which makes H bonds with many additional water molecules. The formula written below shows an example of a formalized formalized formalized formalized formalized.
The hydrated proton is generally presented to illustrate the active function of water and the proton-water interaction [however, for convenience, we occasionally show the unhydrated proton].
Display it as H+(aq)] thus an Arrhenius acid may be defined as a chemical that produces H3O+ in water:
HA(aq) + H2O(l) ⟶ A– (aq) + H3O+(aq) − H + H A + + H O A O H H H
Typical Arrhenius acids include HCl, HNO3, and HCN, whereas typical Arrhenius bases include NaOH, KOH, and Ba(OH)2.
Arrhenius bases have distinct OH ions in their structures because they are ionic compounds, but Arrhenius acids never contain discrete H+ ions. Instead, they have covalently linked H atoms that ionize when acid molecules dissolve in water.
When an acid and a base react, they neutralize each other. As we will see, the definition of this word has evolved, but in the Arrhenius sense, neutralization happens when the H+ from the acid and the OH from the base combine to produce H2O.
Arrhenius was able to explain a fundamental aspect regarding neutralization: no matter which strong acid and a strong base react, and no matter which salt emerges, H°rxn equals 55.9 kJ per molecule.
A mole of water was produced. According to Arrhenius, the enthalpy change is always the same, identical because the reaction always produces the same product—a hydrogen ion and a hydroxide ion in water:
H+(aq) + OH−(aq) ⟶ H2O(l) ΔH°rxn = −55.9 kJ
The dissolved salt that is present, for example, NaCl in the reaction of sodium hydroxide with hydrochloric acid, Na+(aq) + OH−(aq) + H+(aq) + Cl−(aq) ⟶ Na+(aq) + Cl−(aq) + H2O(l) exists as hydrated spectator ions and does not affect ΔH°rxn.
Despite its relevance at the time, the Arrhenius definition quickly revealed its shortcomings. Arrhenius and others discovered that even though some compounds lack distinct OH ions, they nevertheless act as bases. In water, for example, NH3 and K2CO3 both produce OH.
To cover organisms that lack OH– ions in their structures, broader acid-base classifications are necessary. Water binds the proton produced by acid in an aqueous solution to generate a hydrated species denoted by H3O+ (aq).
Acids include H and yield H3O+ in water, bases contain OH and yield OH in water, and an acid-base reaction is the reaction of H+ and OH to generate H2O, according to the Arrhenius definition.
Many chemicals that produce OH ions in water do not include OH in their formulae, which is a significant restriction of the Arrhenius concept. Ammonia, amines, and numerous salts of weak acids, such as NaF, are examples.
Another constraint was that water had to be transported to function as a solvent in acid-base reactions. In the early twentieth century, J. N. Brnsted and others, T. M. Lowry proposed definitions that eliminate these constraints.
According to the Brasted-Lowry acid-base definition, an acid is any substance that provides an H+ ion as a proton donor. H must be present in the formula of an acid; HNO3 and H2PO4 are examples, as they are only two of countless instances. Arrhenius did everything. Brasted-Lowry acids are acids.
A base is any substance that takes an H+ ion as a proton acceptor. A foundation is required, for which they contain a single pair of electrons to bind H+; NH3, CO3 are a few examples.
2, as well as F, as well as the letter OH. Brasted-Lowry bases are not Arrhenius bases, but they are all bases. The Brasted-Lowry base OH is found in Arrhenius bases.

According to this viewpoint, an acid-base reaction happens when one species contributes a proton and another species takes it at the same time: an acid-base reaction is therefore a proton transfer event. Acid-base reactions can occur in gases, nonaqueous solutions, heterogeneous mixtures, and aqueous solutions.
An acid-base reaction happens even when no acid is present, according to this definition, (or a base) simply dissolves in water because water serves as a proton acceptor (or a base).
Water receives a proton from acid, as shown in the image attached below. Water receives a proton from acid (Figure 18.1A). When HCl dissolves in water, it transfers one H+ ion (a proton) from HCl to H2O, where it attaches to a lone pair of electrons on the O atom, creating H3O+. As a result, HCl (the acid) possesses H+ was provided, and H2O (the base) received it:
HCl(g) + H2O(l) Cl −(aq) + H3O+(aq)
A Brasted-Lowry acid-base reaction is the dissolution of an acid or a base in water. A The dissolving of the acid HCl in the base water. B, The acid water dissolves the base NH3.
A proton from water is accepted by the base (Figure 18.1B). When ammonia dissolves in water, and H+ from H2O is transferred to the lone pair of N, resulting in the formation of NH4 + H2O is converted into an OH ion:
NH3(aq) + H2O(l) NH4 +(aq) + OH−(aq)
In this example, H2O (the acid) gave the H+ and NH3 (the base) took it. H2O is amphiprotic, which means it may function as a base (accepts an H+) in one circumstance and an acid (donates an H+) in the other.
Many additional species are also amphiprotic
 Knowt
Knowt
