
Chapter 2 - The Chemistry of Life
The matter that makes up every object consists of one or more elements that organize into atoms and molecules.
Element - a substance that cannot be broken down by chemical means into other substances
Living things are mostly composed of:
carbon
hydrogen
oxygen
nitrogen
phosphorus
sulfur
Periodic Table lists all known elements
Each box shows:
the elements full name
the elements one or two letter symbol
atomic number
atomic weight
mass - things inside
weight - gravitational pull
Atoms make up all matter
Atom - smallest piece of an element that retains the characteristics of the element
composed of protons, neutrons, and electrons
electrons - surround the atomic nucleus
protons and neutrons - in atomic nucleus
protons - positively charged (+), mass 1
neutrons - neutral (0) mass 1
electrons - negatively charged (-), mass 0
Atomic number - number of protons
Atom mass number - number of protons and neutrons
Ion - charged atom
Atoms do not have charge
Isotopes are different forms of the same element
The number of neutrons may vary which causes each isotope to have different masses.
The atomic weight on the periodic table is the average mass
Chemical Bonds link atoms together
Atoms are organized into molecules
Chemical bonds are determined by electrons
The number and distribution of electrons around an atom determines whether how the atom will react with other atoms
Energy shells, orbitals, contain the atoms electrons
The shell farthest from the nucleus is the most important for bonding
Electrons are arranged in pairs on the shells
Unpaired electrons form bonds with other atoms
Atoms are more stable when their outer shells have no vacancies
The more vacancies, the more likely they are to bond with another atom
Covalent Bonds - when atoms share electrons
Double bonds - share 4 electrons between atoms
Noble gases - full and do not bond
Electronegativity measures an atom’s ability to attract electrons
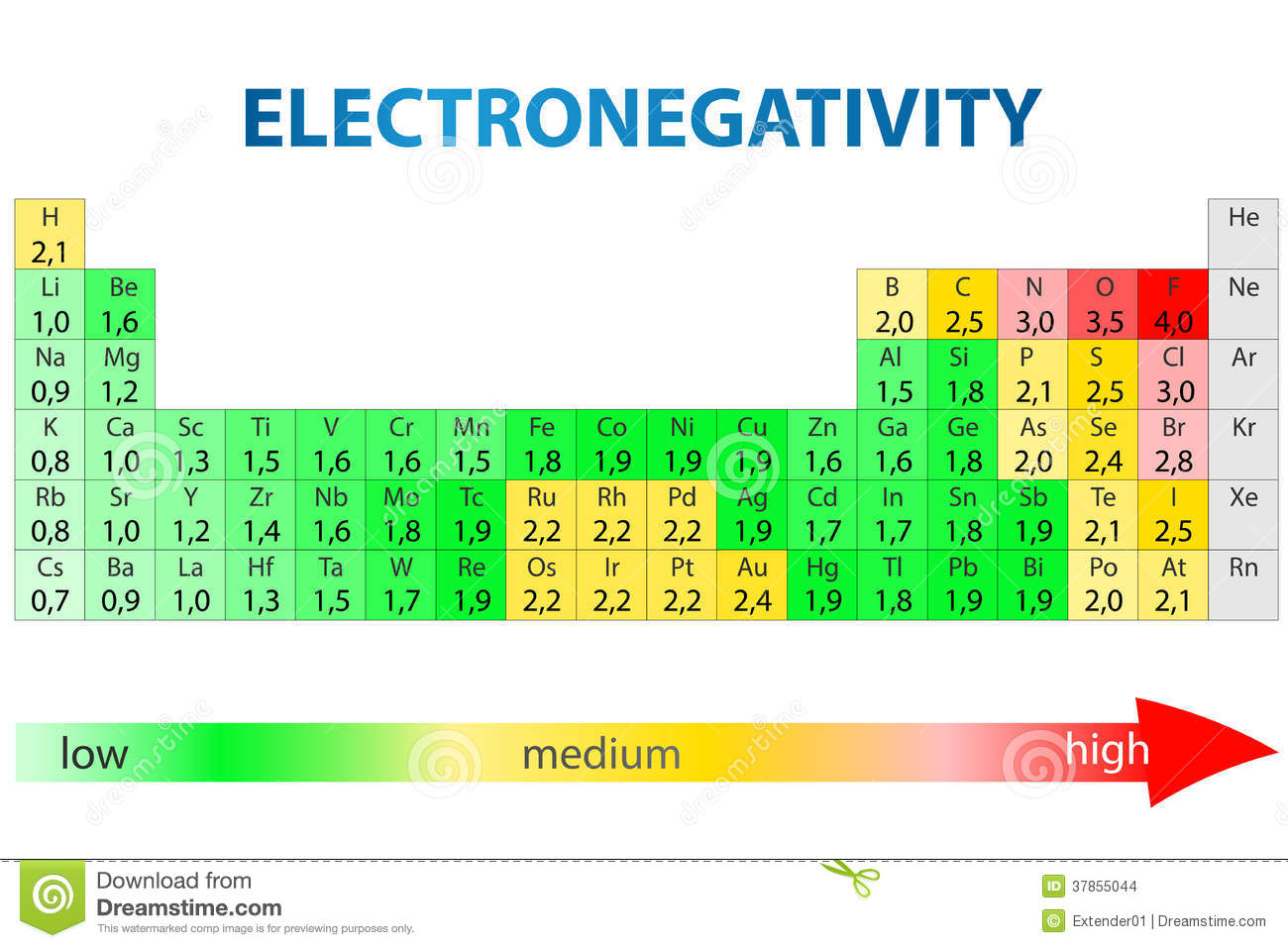
Differences determine chemical bonds
The periodic table arranges atoms by electronegativity
When atoms have similar electronegativity, neither will pull electrons more strongly than the other
Nonpolar covalent bonds form during this
Some atoms have a partial charge
Electrons spend more time - slightly negative charge (atom)
Electrons spend less time - slightly positive charge (atom)
This charge difference gives the bonds polarity
Different electronegativity - polar covalent bonds
Polarity in water molecules creates hydrogen bonds
Slightly positive charge on the hydrogen atom attracts the negatively charge of a neighboring water molecule
Hydrogen bonds pull water molecules close to each other
This gives water unique properties
This is important to DNA structures and proteins
Very high electronegativity forms ionic bonds
Some bonds have such different electronegativities that one atom completely pulls an electron away from each other
electrons can completely transfer over to highly electronegative atoms
atom that loses an electron - positively charged
atom that gains - negatively charged
charge difference attracts the atoms to each other, forming ionic bonds
In ionic bonds, both atoms get full outer shells, so both atoms become stable
Type | Chemical Basis | Strength | Example |
|---|---|---|---|
Ionic Bonds | Atoms with electronegative differences (large >1.7); one atom takes an electron from another. Opposingly charged ions attract each other | Strong but breaks in water | Sodium Chloride (NaCl) |
Covalent Bond | Two atoms share a pair of electrons | Strong | |
nonpolar | Electronegativity differences are small (<0.4) | H - H bond in hydrogen bond | |
polar | electronegativity difference is moderate or large (0.4 - 1.7) | O - H bond in water molecule | |
Hydrogen bond | Partially negative atom attracts a hydrogen atom with partially positive charged. Hydrogen bonds form between adjacent molecules or between different parts of a large molecule | Weak | Attraction between adjacent water molecules |
Two elements with similar/moderately different electronegativities will - form nonpolar covalent bonds
Two elements with very different electronegativities will - form ionic bonds
Water Unique Properties
Cohesion - tendency of water molecules to stick to one another
give high surface tension
Adhesion - allows water molecules to form hydrogen bonds with other molecules
Cohesion and Adhesion allows water to “climb” from a trees roots to its highest leaves
Water is an excellent solvent
Water dissolves hydrophilic (water - loving) substances
polar solutes and ions
The polarity of water molecules help water dissolve most biologically important molecules
Salt (NaCl)
Slight negative charge on water attracts positive charges (Na+)
Slight positive charges on water attracts negative charges (Cl-)
Only dissolved selected molecules
Water does not dissolve hydrophobic (water - fearing) solutes
Example: Lipids (butter) that have nonpolar covalent bonds
Water regulates temperature
Hydrogen bonds make water resist changes in temperature
water cools and heats up slowly
This is how sweating cools the body
Water expands when it freezes
Hydrogen bonds make water molecules spread out as the water freezes into ice
This is why ice is less dense than liquid water and ice floats to the top
In large bodies of water, a top layer of ice provides insulation to keep aquatic life and everything else from freezing underneath
Water participates in chemical reactions needed for life
Water is a reactant in reactions that build and break down all classes of biological molecules
pH scale shows the amount of H+ ions in solutions
Acidic pH <7 - the lower the pH, the stronger the acid
Neutral pH =7
Alkaline (basic) pH >7 - the higher the pH, the stronger the base
Acidic solutions have a low pH and a high H+ concentration
Basic solutions have a high pH and a low H+ concentration
Have more OH- ions than H+ ions
Many organisms maintain pH homeostasis close to pH=7
If an organism stray too far from its optimal pH, it could die
Buffer solutions - maintain a constant pH by absorbing or releasing H+ into a solution
pH too high - releases H+ to lower pH
pH too low - absorbs H+ to raise pH
Organic Molecules
Contain both carbon and hydrogen (ex: methane)
They are biologically important
they are needed for life’s processes and are categorized into four main categories
Carbohydrates
Proteins
Nucleic acids
Lipids
Organic molecules such as carbohydrates, proteins, and fats are common in our diets.
Type of Molecules | Chemical Structure | Functions |
|---|---|---|
Carbohydrates | ||
simple sugars | Monosaccharides and disaccharides | Provide quick energy |
complex carbohydrates (cellulose, chitin, starch, glycogen) | Polysaccharides (Polymers of monosaccharides) | Support cells and organisms (cellulose, chitin), store energy (starch, glycogen) |
Proteins | Polymers of amino acids | Carry out nearly all of the work of the cell |
Nucleic Acids (DNA, RNA) | Polymers of nucleotides | Store and use genetic information and transmit it to the next generation |
Lipids | Diverse, hydrophobic | |
Triglycerides (fats) | Glycerol + 3 fatty acids | Store energy |
Phispholides | Glycerol + 2 fatty acids + phosphate group | Form major part of biological membranes |
Steroids | Four fused rings, mostly of C and H | Stabilize animal membranes; sex hormones |
Waxes | Fatty acids + other hydrocarbons or alcohols | Provides waterproofing |
Organic Molecules are made of monomers
monomers - single unit of carbohydrate, protein, or nucleic acid
form together to form polymers
Dehydration synthesis - chemical reaction that joins monomers
enzymes form bonds between two monomers
water is released as a part of the reaction
Hydrolysis - chemical reactions that break polymers apart
enzymes break bonds between monomers
water molecule is required
Carbohydrates include simple sugars and polysaccharides
Monosaccharides are simple sugars; they are the monomers that make up larger carbohydrates
example: ribose, glucose, and fructose
Dehydration synthesis binds two simple sugars together, forming a disaccharide
ex: sucrose
Hydrolysis breaks disaccharides into monosaccharides
Complex Carbohydrates
Polysaccharides are long chains of carbohydrates
cellulose: structure
starch: energy
glycogen: energy
Proteins are made of amino acids
Monomers of proteins are amino acids
Dehydration synthesis binds two amino acids together, forming a dipeptide
A long chain of amino acids is called a polypeptide
Hydrolysis separates dipeptides and polypeptides into individual amino acids
Proteins have many different structures and functions
Proteins are the “workers” of the cell; they do almost everything
Collagen - Create cellular structures
Actin and Myosin - Produce muscle contractions
Each amino acid has its own chemical and physical properties
The 20 different amino acids have 20 different R - groups
These properties in turn determine the properties of the proteins
Polypeptides fold up into proteins
A chain of amino acids folds up into a unique 3 - D shape to become a protein
The function of a protein depends on its shape, or tertiary structure
Denatured proteins lose their shape
Proteins Structures
Primary structure (sequence): Amino acid sequence of a polypeptide
Secondary structure (“substructure): Localized area of coils, sheets, and loops within a polypeptide
Tertiary structure (polypeptide structure): Overall shape of one polypeptide
Quaternary structure (protein shape): Overall protein shape, arising from interaction between the multiple polypeptides that make up the functional protein. Only proteins with multiple polypeptides have quaternary structure
Nucleic Acids carry genetic information
Nucleic acids include DNA and RNA
DNA : Deoxynucleic Acid
RNA: Ribonucleic Acid
The primary structure of each protein in a cell is determined by the nucleic acids
The monomers of nucleic acids are nucleotides
The 3 different parts of a nucleotide are a phosphate group, a 5 - carbon sugar and a nitrogenous base
There are 5 possible nitrogenous basesz
DNA + RNA both incorporate Adenine, Cytosine, Guanine
Only DNA - Thymine
Only RNA - Uracil
Dehydration synthesis binds two nucleotides which creates DNA and RNA
Hydrolysis separates nucleic acids into individual nucleotides
DNA Replication
Happens in the nucleus
DNA has to uncoil
Hydrogen bonds break
Sides separate
Floating nucleotides come in from the cytoplasm
Hydrogen bonds form
DNA recoils
2 DNA molecules as the result
Lipids are a collection of different hydrophobic molecules
All lipids are hydrophobic
Different groups of lipids include molecules with varying structure and function
THESE ARE NOT BUILT FROM CHAINS OF MONOMERS
Classes of Lipids
Triglycerides (fats and oils) - energy rich, needed for long term energy storage
formed by covalently attaching 3 fatty acid molecules to a glycerol molecule
Dehydration synthesis links fatty acids to glycerol
Hydrolysis separates fatty acids from glycerol
Some fatty acids are saturated
All carbons of a saturated fatty acids are bonded to 4 other atoms which has a straight shape
Same fatty acids are unsaturated which contain at least one double bond and has a bent shape
Saturation gives triglycerides different properties
Bends in the unsaturated fatty acids prevent them from packing close together so unsaturated fats like oils are therefore liquids at room temperature
Waxes - second class of lipids
Composed of fatty acids combined with alcohols
Particularly hydrophobic
In nature, waxes from waterproof seals
Steroids - third class of lipids
Cholesterol regulates the fluidity of animal cell membranes; it is also used to synthesize many sex hormones
Check flow chart in notes
Chapter 2 - The Chemistry of Life
The matter that makes up every object consists of one or more elements that organize into atoms and molecules.
Element - a substance that cannot be broken down by chemical means into other substances
Living things are mostly composed of:
carbon
hydrogen
oxygen
nitrogen
phosphorus
sulfur
Periodic Table lists all known elements
Each box shows:
the elements full name
the elements one or two letter symbol
atomic number
atomic weight
mass - things inside
weight - gravitational pull
Atoms make up all matter
Atom - smallest piece of an element that retains the characteristics of the element
composed of protons, neutrons, and electrons
electrons - surround the atomic nucleus
protons and neutrons - in atomic nucleus
protons - positively charged (+), mass 1
neutrons - neutral (0) mass 1
electrons - negatively charged (-), mass 0
Atomic number - number of protons
Atom mass number - number of protons and neutrons
Ion - charged atom
Atoms do not have charge
Isotopes are different forms of the same element
The number of neutrons may vary which causes each isotope to have different masses.
The atomic weight on the periodic table is the average mass
Chemical Bonds link atoms together
Atoms are organized into molecules
Chemical bonds are determined by electrons
The number and distribution of electrons around an atom determines whether how the atom will react with other atoms
Energy shells, orbitals, contain the atoms electrons
The shell farthest from the nucleus is the most important for bonding
Electrons are arranged in pairs on the shells
Unpaired electrons form bonds with other atoms
Atoms are more stable when their outer shells have no vacancies
The more vacancies, the more likely they are to bond with another atom
Covalent Bonds - when atoms share electrons
Double bonds - share 4 electrons between atoms
Noble gases - full and do not bond
Electronegativity measures an atom’s ability to attract electrons

Differences determine chemical bonds
The periodic table arranges atoms by electronegativity
When atoms have similar electronegativity, neither will pull electrons more strongly than the other
Nonpolar covalent bonds form during this
Some atoms have a partial charge
Electrons spend more time - slightly negative charge (atom)
Electrons spend less time - slightly positive charge (atom)
This charge difference gives the bonds polarity
Different electronegativity - polar covalent bonds
Polarity in water molecules creates hydrogen bonds
Slightly positive charge on the hydrogen atom attracts the negatively charge of a neighboring water molecule
Hydrogen bonds pull water molecules close to each other
This gives water unique properties
This is important to DNA structures and proteins
Very high electronegativity forms ionic bonds
Some bonds have such different electronegativities that one atom completely pulls an electron away from each other
electrons can completely transfer over to highly electronegative atoms
atom that loses an electron - positively charged
atom that gains - negatively charged
charge difference attracts the atoms to each other, forming ionic bonds
In ionic bonds, both atoms get full outer shells, so both atoms become stable
Type | Chemical Basis | Strength | Example |
|---|---|---|---|
Ionic Bonds | Atoms with electronegative differences (large >1.7); one atom takes an electron from another. Opposingly charged ions attract each other | Strong but breaks in water | Sodium Chloride (NaCl) |
Covalent Bond | Two atoms share a pair of electrons | Strong | |
nonpolar | Electronegativity differences are small (<0.4) | H - H bond in hydrogen bond | |
polar | electronegativity difference is moderate or large (0.4 - 1.7) | O - H bond in water molecule | |
Hydrogen bond | Partially negative atom attracts a hydrogen atom with partially positive charged. Hydrogen bonds form between adjacent molecules or between different parts of a large molecule | Weak | Attraction between adjacent water molecules |
Two elements with similar/moderately different electronegativities will - form nonpolar covalent bonds
Two elements with very different electronegativities will - form ionic bonds
Water Unique Properties
Cohesion - tendency of water molecules to stick to one another
give high surface tension
Adhesion - allows water molecules to form hydrogen bonds with other molecules
Cohesion and Adhesion allows water to “climb” from a trees roots to its highest leaves
Water is an excellent solvent
Water dissolves hydrophilic (water - loving) substances
polar solutes and ions
The polarity of water molecules help water dissolve most biologically important molecules
Salt (NaCl)
Slight negative charge on water attracts positive charges (Na+)
Slight positive charges on water attracts negative charges (Cl-)
Only dissolved selected molecules
Water does not dissolve hydrophobic (water - fearing) solutes
Example: Lipids (butter) that have nonpolar covalent bonds
Water regulates temperature
Hydrogen bonds make water resist changes in temperature
water cools and heats up slowly
This is how sweating cools the body
Water expands when it freezes
Hydrogen bonds make water molecules spread out as the water freezes into ice
This is why ice is less dense than liquid water and ice floats to the top
In large bodies of water, a top layer of ice provides insulation to keep aquatic life and everything else from freezing underneath
Water participates in chemical reactions needed for life
Water is a reactant in reactions that build and break down all classes of biological molecules
pH scale shows the amount of H+ ions in solutions
Acidic pH <7 - the lower the pH, the stronger the acid
Neutral pH =7
Alkaline (basic) pH >7 - the higher the pH, the stronger the base
Acidic solutions have a low pH and a high H+ concentration
Basic solutions have a high pH and a low H+ concentration
Have more OH- ions than H+ ions
Many organisms maintain pH homeostasis close to pH=7
If an organism stray too far from its optimal pH, it could die
Buffer solutions - maintain a constant pH by absorbing or releasing H+ into a solution
pH too high - releases H+ to lower pH
pH too low - absorbs H+ to raise pH
Organic Molecules
Contain both carbon and hydrogen (ex: methane)
They are biologically important
they are needed for life’s processes and are categorized into four main categories
Carbohydrates
Proteins
Nucleic acids
Lipids
Organic molecules such as carbohydrates, proteins, and fats are common in our diets.
Type of Molecules | Chemical Structure | Functions |
|---|---|---|
Carbohydrates | ||
simple sugars | Monosaccharides and disaccharides | Provide quick energy |
complex carbohydrates (cellulose, chitin, starch, glycogen) | Polysaccharides (Polymers of monosaccharides) | Support cells and organisms (cellulose, chitin), store energy (starch, glycogen) |
Proteins | Polymers of amino acids | Carry out nearly all of the work of the cell |
Nucleic Acids (DNA, RNA) | Polymers of nucleotides | Store and use genetic information and transmit it to the next generation |
Lipids | Diverse, hydrophobic | |
Triglycerides (fats) | Glycerol + 3 fatty acids | Store energy |
Phispholides | Glycerol + 2 fatty acids + phosphate group | Form major part of biological membranes |
Steroids | Four fused rings, mostly of C and H | Stabilize animal membranes; sex hormones |
Waxes | Fatty acids + other hydrocarbons or alcohols | Provides waterproofing |
Organic Molecules are made of monomers
monomers - single unit of carbohydrate, protein, or nucleic acid
form together to form polymers
Dehydration synthesis - chemical reaction that joins monomers
enzymes form bonds between two monomers
water is released as a part of the reaction
Hydrolysis - chemical reactions that break polymers apart
enzymes break bonds between monomers
water molecule is required
Carbohydrates include simple sugars and polysaccharides
Monosaccharides are simple sugars; they are the monomers that make up larger carbohydrates
example: ribose, glucose, and fructose
Dehydration synthesis binds two simple sugars together, forming a disaccharide
ex: sucrose
Hydrolysis breaks disaccharides into monosaccharides
Complex Carbohydrates
Polysaccharides are long chains of carbohydrates
cellulose: structure
starch: energy
glycogen: energy
Proteins are made of amino acids
Monomers of proteins are amino acids
Dehydration synthesis binds two amino acids together, forming a dipeptide
A long chain of amino acids is called a polypeptide
Hydrolysis separates dipeptides and polypeptides into individual amino acids
Proteins have many different structures and functions
Proteins are the “workers” of the cell; they do almost everything
Collagen - Create cellular structures
Actin and Myosin - Produce muscle contractions
Each amino acid has its own chemical and physical properties
The 20 different amino acids have 20 different R - groups
These properties in turn determine the properties of the proteins
Polypeptides fold up into proteins
A chain of amino acids folds up into a unique 3 - D shape to become a protein
The function of a protein depends on its shape, or tertiary structure
Denatured proteins lose their shape
Proteins Structures
Primary structure (sequence): Amino acid sequence of a polypeptide
Secondary structure (“substructure): Localized area of coils, sheets, and loops within a polypeptide
Tertiary structure (polypeptide structure): Overall shape of one polypeptide
Quaternary structure (protein shape): Overall protein shape, arising from interaction between the multiple polypeptides that make up the functional protein. Only proteins with multiple polypeptides have quaternary structure
Nucleic Acids carry genetic information
Nucleic acids include DNA and RNA
DNA : Deoxynucleic Acid
RNA: Ribonucleic Acid
The primary structure of each protein in a cell is determined by the nucleic acids
The monomers of nucleic acids are nucleotides
The 3 different parts of a nucleotide are a phosphate group, a 5 - carbon sugar and a nitrogenous base
There are 5 possible nitrogenous basesz
DNA + RNA both incorporate Adenine, Cytosine, Guanine
Only DNA - Thymine
Only RNA - Uracil
Dehydration synthesis binds two nucleotides which creates DNA and RNA
Hydrolysis separates nucleic acids into individual nucleotides
DNA Replication
Happens in the nucleus
DNA has to uncoil
Hydrogen bonds break
Sides separate
Floating nucleotides come in from the cytoplasm
Hydrogen bonds form
DNA recoils
2 DNA molecules as the result
Lipids are a collection of different hydrophobic molecules
All lipids are hydrophobic
Different groups of lipids include molecules with varying structure and function
THESE ARE NOT BUILT FROM CHAINS OF MONOMERS
Classes of Lipids
Triglycerides (fats and oils) - energy rich, needed for long term energy storage
formed by covalently attaching 3 fatty acid molecules to a glycerol molecule
Dehydration synthesis links fatty acids to glycerol
Hydrolysis separates fatty acids from glycerol
Some fatty acids are saturated
All carbons of a saturated fatty acids are bonded to 4 other atoms which has a straight shape
Same fatty acids are unsaturated which contain at least one double bond and has a bent shape
Saturation gives triglycerides different properties
Bends in the unsaturated fatty acids prevent them from packing close together so unsaturated fats like oils are therefore liquids at room temperature
Waxes - second class of lipids
Composed of fatty acids combined with alcohols
Particularly hydrophobic
In nature, waxes from waterproof seals
Steroids - third class of lipids
Cholesterol regulates the fluidity of animal cell membranes; it is also used to synthesize many sex hormones
Check flow chart in notes
 Knowt
Knowt
