
Chapter 2: The Chemical Basis of Life
2.1 Atoms and Atomic Bonds
Everything is made of matter, including humans.
Matter
Anything that takes up space and has mass.
Matter can exist as a solid, liquid, gas, or plasma.
Matter is composed of elements
Element
A substance that cannot be broken down into other substances by ordinary chemical means.
There are only 92 naturally occurring elements, and each of these differs from the others in its chemical or physical properties, such as density, solubility, melting point, and reactivity.
Although all elements are present on Earth, the proportion of each element differs between living organisms and nonliving things.
Four elements—carbon, hydrogen, nitrogen, and oxygen—make up about 96% of the body weight of most organisms.
Elements such as phosphorus, calcium, and sulfur may also be found in abundance in living organisms.
Minerals, such as zinc and chromium, are found at very low, or trace, levels in organisms.
Where do Elements Come From?
The majority of the heavier elements, such as iron, are produced only by the intense chemical and physical reactions within stars.
When supernovas occur, they scatter heavier elements into space, where they eventually are involved in the formation of planets.
The late astronomer and philosopher Carl Sagan frequently referred to humans as “star stuf.”
Atomic Structure
Atomic theory
A theory that states that elements consist of tiny particles called atoms.
Each element consists of only one kind of atom; it is because of this the same name is given to an element and its atoms; This name is represented by one or two letters, called the atomic symbol.
For example, the symbol H stands for an atom of hydrogen, and the symbol Na (for natrium in Latin) stands for an atom of sodium.
A single atom is made mostly of three types of subatomic particles, which include the following:
Neutrons
Contain no electrical charge.
Protons
Contain a positive charge.
Electrons
Contain a negative charge.
Protons and neutrons are located within the center of an atom, which is called the nucleus.
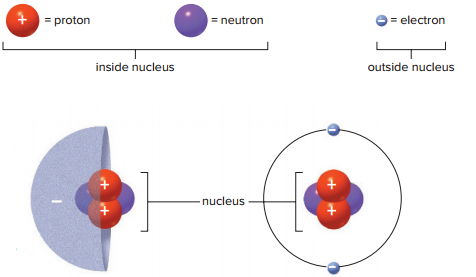
Electrons are in a constant state of motion, so their estimated location is often shown as a cloud.
Most of an atom is empty space.
In an atom, the mass is just about equal to the sum of its protons and neutrons.
Protons and neutrons are assigned one mass unit each.
Electrons, being matter, have mass, but they are so small that their mass is assumed to be zero in most calculations.
The term mass is used rather than weight because mass is constant, but weight is associated with gravity and thus varies depending on an object's location in the universe.
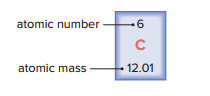
The number of protons makes an atom unique and may be used to identify which element the atom belongs to.
This is called the atom’s atomic number.
All atoms of an element have the same number of protons.
The number of neutrons may vary between atoms of an element.
Atomic mass
The average of the mass numbers of atoms.
The atomic number tells you the number of positively charged protons.
If the atom is electrically neutral, then the atomic number also indicates the number of negatively charged electrons.
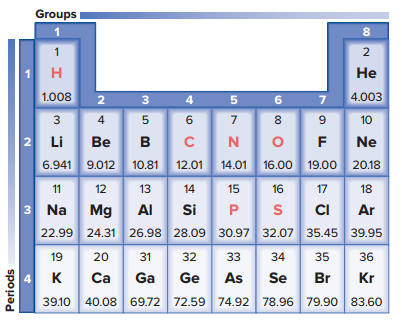
Once chemists discovered a number of the elements, they began to realize that the elements’ chemical and physical characteristics recur in a predictable manner.
In a periodic table, the atomic number is written above the atomic symbol, and the atomic mass is written below the atomic symbol.
Noble gases
Gases that rarely react with another atom, such as helium (He) and neon (Ne)
Isotopes
Isotopes
Atoms of the same element that differ in the number of neutrons.
A nucleus with excess neutrons is unstable and may decay and emit radiation; Although
Such an isotope is said to be radioactive
not all isotopes are radioactive.
The radiation given off by radioactive isotopes can be detected in various ways, including a Geiger counter.
Uses of Radioactive Isotopes
The importance of chemistry to biology and medicine is nowhere more evident than in the many uses of radioactive isotopes, including:
As tracers to detect molecular changes or to destroy abnormal or infectious cells.
Positron-emission tomography (PET).
Radioactive substances in the environment can cause harmful chemical changes in cells, damage DNA, and cause cancer.
The release of radioactive particles following a nuclear power plant accident can have far-reaching and long-lasting effects on human health.
But the effects of radiation can also be put to good use, such as in the following examples:
Packets of radioactive isotopes can be placed in the body so that the subatomic particles emitted destroy only cancer cells, with little risk to the rest of the body.
Radiation from radioactive isotopes has been used for many years to sterilize medical and dental equipment.
Mail that is destined for the White House and congressional offices in Washington, DC, is irradiated to protect against dangerous biological agents, such as anthrax.
Arrangement of Electrons in an Atom
The electrons of an atom are constantly moving; Although it is not possible to determine the precise location of an individual electron at any given moment, it is helpful to construct models of atoms that show electrons at discrete energy levels about the nucleus.
The discrete energy levels are known as Electron shells
Electrons orbit the nucleus at particular energy levels called electron shells. The first shell contains up to two electrons, and each shell thereafter has an increasing number of electrons.
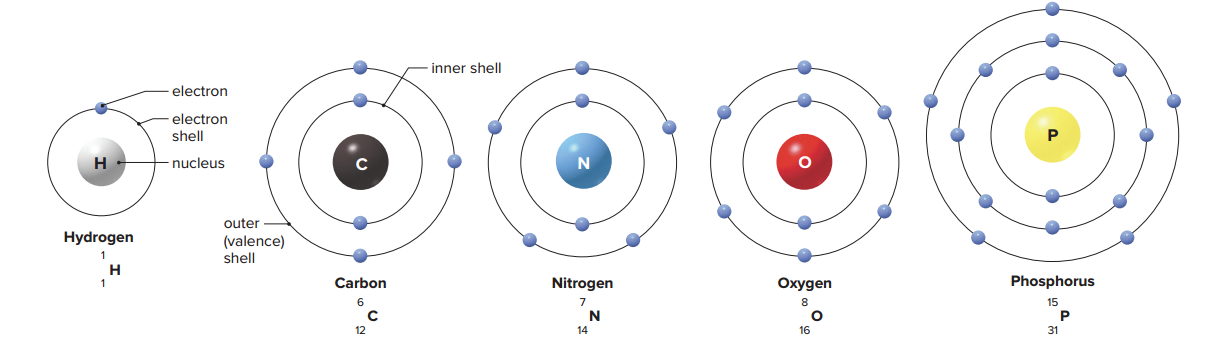
Each electron shell contains a certain number of electrons.
Since the nucleus of an atom is positively charged, negatively charged electrons require an increasing amount of energy to push them farther away from the nucleus; It is because of this that Electrons in outer electron shells contain more energy than those in inner electron shells.
If an atom has only one shell, the outer shell is complete when it has two electrons; If an atom has two or more shells, the outer shell is most stable when it has eight electrons; this is called the octet rule.
Atoms with fewer than eight electrons in the outer shell react with other atoms in such a way that each has a complete outer shell after the reaction.
When atoms give up, accept, or share electrons in order to have eight electrons in the outer shell; This is known as the valence shell.
The valence shell determines chemical reactivity.
The size of an atom is also important.
Suppose carbon (C) and silicon (Si) are both in groups of 4. In that case, that means they can bond to achieve 8 electrons, but if you have C with 2 shells and Si with 3 shells, the smaller carbon atom can bond with other carbon atoms to form long-chained molecules, but the silicon atom is unable to bond.
The chemical properties of atoms—that is, the ways they react—are primarily determined by the arrangement of their electrons.
Types of Chemical Bonds
Molecule
A group of atoms bonded together.
Compound
When a molecule contains atoms of more than one element.
Compounds and molecules contain two primary types of chemical bonds, those being the following:
Ionic
Covalent
The type of bond that forms depends on whether two bonded atoms share electrons or whether one has given electrons to the other.
Ionic Bonding
An ionic bond forms when two atoms are held together by the attraction between opposite charges.
Sodium (Na), with only one electron in its third shell, usually gives up an electron; thus,
once it does so, the second shell, with eight electrons, becomes its outer shell.
Chlorine (CI) tends to take on an electron because its outer shell has seven electrons; If chlorine gets one more electron, it has a completed outer shell, so when a sodium atom and a chlorine atom react; an electron is transferred from sodium to chlorine, and both atoms now have eight electrons in their outer shells.
The sodium ion has one more proton than it has electrons; therefore, it has a net charge of +1 (symbolized by Na+).
The chloride ion has one more electron than it has protons; therefore, it has a net charge of –1 (symbolized by Cl–).
This electron transfer causes these atoms to become ions or charged atoms.
Negatively charged ions often have names that end in “ide,” and thus, Cl– is called a chloride ion.
Ionic compounds are often found as salts, solid substances that usually separate and exist as individual ions in water.
An example is sodium chloride (NaCl) or table salt.
Covalent Bonding
A covalent bond results when two atoms share electrons in order to have a completed outer shell.
In a hydrogen atom, the outer shell is complete when it contains two electrons;
If hydrogen is in the presence of a strong electron acceptor, it gives up its electron to become a hydrogen ion (H+).
If this is not possible, hydrogen can share with another atom and thereby have a completed outer shell.
Scientists opt to use structural formulas instead of complete model drawings since a structural formula is simpler.
Example: H--H
The straight line is used to indicate a pair of shared electrons.
A molecular formula omits the lines that indicate bonds and shows the number of atoms involved.
H2.
Sometimes, atoms share more than two electrons to complete their octets.
A double covalent bond occurs when two atoms share two pairs of electrons.
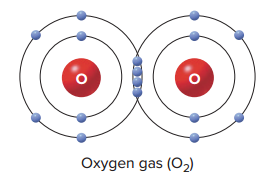
If writing a double covalent bond in structural formula, it would appear as the following example.
O==O
It is also possible for atoms to form triple covalent bonds, as in nitrogen gas (N2), which can be written as the following:
N≡N
Single covalent bonds between atoms are pretty strong, but double and triple bonds are even stronger.
A single atom may form bonds with more than one other atom.
Chemical Formulas and Reactions
Chemical reactions are very important to organisms.
In a chemical reaction, the molecules are often represented by a chemical formula.
Chemical formulas do not indicate the arrangement of these elements; in fact,
they are sometimes several different structures that can be formed based on a chemical formula.
Reactants
Molecules that participate in a reaction.
Products
Molecules are formed by a reaction.
2.2 Water’s Importance to Life
All organisms are 70–90% water; their cells consist of membranous compartments enclosing aqueous solutions.
The structure of a water molecule gives it unique properties, properties that play an essential role in how living organisms function.
The Structure of Water
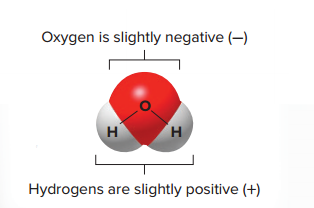
The electrons shared between two atoms in a covalent bond are not always shared equally.
Atoms differ in their electronegativity—that is, their affinity for electrons in a covalent bond.
Atoms that are more electronegative tend to hold shared electrons more tightly than do those that are less electronegative; this
unequal sharing of electrons causes the bond to become polar.
Polar
Atoms on both sides of the bond are partially charged even though the overall molecule itself bears no net charge.
The polarity of water molecules causes them to be attracted to one another; Positive hydrogen atoms in one molecule are attracted to the negative oxygen atoms in other water molecules.
This attraction is called a hydrogen bond.
Each water molecule can engage in as many as four hydrogen bonds
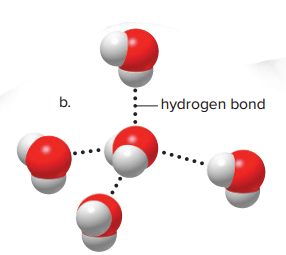
The covalent bond is much stronger than a hydrogen bond, but the large number of hydrogen bonds in water makes for a strong attractive force.
The properties of water are due to its polarity and its ability to form hydrogen bonds.
Properties of Water
The properties of water that support life are the following:
Solvency
Cohesion
Adhesion
High surface tension
High heat capacity
Varying density
Water is a Solvent
Due to waters polarity and hydrogen-bonding ability, water dissolves a significant number of substances.
Hydrophilic
Molecules that are attracted to water.
Hydrophobic
Nonionized and nonpolar molecules that are not attracted to water.
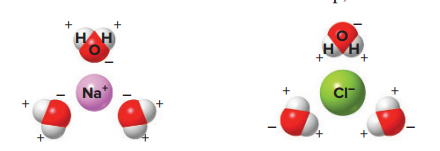
When a salt such as sodium chloride (NaCl) is put into water, the negative ends of the water molecules are attracted to the sodium ions, and the positive ends of the water molecules are attracted to the chloride ions; This attraction causes the sodium ions and the chloride ions to break up, or dissociate, in water.
Water may also dissolve polar nonionic substances, such as long chains of glucose, by forming hydrogen bonds with them.
When ions and molecules disperse in water, they move about and collide, allowing reactions to occur.
Water Molecules Are Cohesive and Adhesive
Cohesion
The ability of water molecules to cling to each other due to hydrogen bonding.
Because of cohesion, water exists as a liquid under ordinary conditions of temperature and pressure.
Adhesion
The ability of water molecules to cling to other polar surfaces.
Adhesion is due to water’s polarity; the positive and negative poles of water molecules cause them to adhere to other polar surfaces.
Due to cohesion and adhesion, liquid water is an excellent transport system.
Both within and outside the cell, water assists in the transport of nutrients and waste materials.
The liquid portion of blood, which transports dissolved and suspended substances within the body, is 90% water.
The cohesion and adhesion of water molecules allow blood to fill the tubular vessels of the cardiovascular system, making transport possible.
Cohesion and adhesion also contribute to the transport of water in plants.
Water Has a High Surface Tension
Because the water molecules at the surface are more strongly attracted to each other than to the air above, water molecules at the surface cling tightly to each other, and that is why we say that water exhibits surface tension.
The stronger the force between molecules in a liquid, the greater the surface tension.
Hydrogen bonding is the main force that causes water to have high surface tension.
Water Has A High Heat Capacity
The many hydrogen bonds that link water molecules allow water to absorb heat without greatly changing in temperature.
Water’s high heat capacity is essential for all organisms
Because the temperature of water rises and falls slowly, terrestrial organisms are better able to maintain their normal internal temperatures and are protected from rapid temperature changes.
Water also has a high heat of vaporization: It takes a great deal of heat to break the hydrogen bonds in water so that it becomes gaseous and evaporates into the environment.
Because of water’s high heat capacity and high heat of vaporization, temperatures along the majority of Earth’s coasts are moderate.
Ice is Less Dense Than Water
Unlike other substances, water expands as it freezes; Since water expands as it freezes, ice is less dense than liquid water, and therefore ice floats on liquid water.
2.3 Acids and Bases
Acidic Solutions (High H+ Concentration)
Acidic solutions have a sharp or sour taste, and therefore we sometimes associate them with indigestion.
Acids
Substances that dissociate in water release hydrogen ions (H+).
Acidic solutions have a higher concentration of H+ ions than OH– ions.
The acidity of a substance depends on how fully it dissociates in water.
HCl dissociates almost completely; therefore, it is called a strong acid.
Basic Solutions (Low H+ Concentration)
Milk of magnesia and ammonia are common basic (alkaline) solutions that most people are familiar with.
Basic solutions have a bitter taste and feel slippery when in water.
Bases
substances that either take up hydrogen ions (H+ ) or release hydroxide ions (OH– ).
For example, an important base is sodium hydroxide (NaOH), which dissociates in this manner.
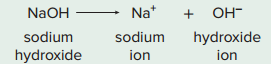
Basic solutions have a higher concentration of OH– ions than H+ ions. Like acids, the strength of a base is determined by how fully it dissociates; the dissociation of sodium hydroxide is almost complete; therefore, it is called a strong base.
Many strong bases, such as ammonia, are useful household cleansers.
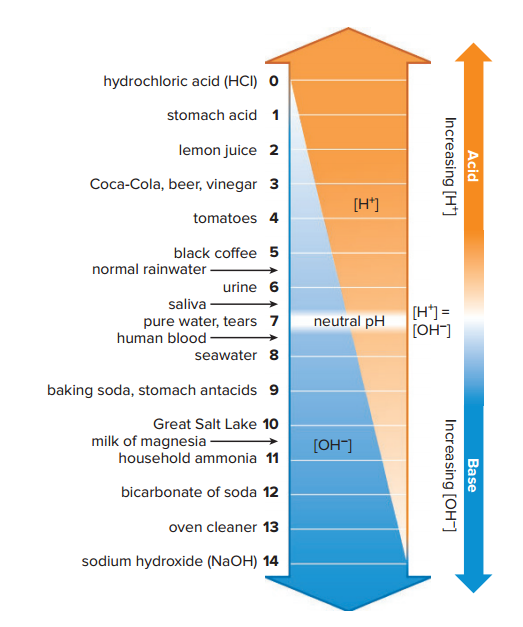
pH and the pH Scale
pH1 is a mathematical way of indicating the number of hydrogen ions in a solution.
The pH scale is used to indicate the acidity or basicity of a solution.
The pH scale ranges from 0 to 14, where 7 represents neutrality, below 7 equals acidic due to the hydrogen ion concentration, and above 7 is basic because [OH–] is greater than [H+ ].
Moving down the pH scale from pH 7 to pH 0, each unit has 10 times the acidity [H+ ] of the previous unit, similarly As we move up the scale from 7 to 14, each unit has 10 times the basicity [OH– ] of the previous unit.
The pH scale was devised to eliminate the use of cumbersome numbers.
The effect of pH on organisms is dramatically illustrated by the phenomenon known as acid precipitation.
Buffers and pH
Buffer
A chemical or a combination of chemicals that keeps pH within established limits.
Buffers resist pH changes because they can take up excess hydrogen ions (H+ ) or hydroxide ions (OH– ).
In the human body, pH needs to be kept within a narrow range in order to maintain homeostasis.
Diseases such as diabetes and congestive heart failure may bring on a condition called acidosis, in which the body is unable to buffer the excessive production of H+ ions.
If left untreated, acidosis can cause a number of health problems and may result in coma or death.
Chapter 2: The Chemical Basis of Life
2.1 Atoms and Atomic Bonds
Everything is made of matter, including humans.
Matter
Anything that takes up space and has mass.
Matter can exist as a solid, liquid, gas, or plasma.
Matter is composed of elements
Element
A substance that cannot be broken down into other substances by ordinary chemical means.
There are only 92 naturally occurring elements, and each of these differs from the others in its chemical or physical properties, such as density, solubility, melting point, and reactivity.
Although all elements are present on Earth, the proportion of each element differs between living organisms and nonliving things.
Four elements—carbon, hydrogen, nitrogen, and oxygen—make up about 96% of the body weight of most organisms.
Elements such as phosphorus, calcium, and sulfur may also be found in abundance in living organisms.
Minerals, such as zinc and chromium, are found at very low, or trace, levels in organisms.
Where do Elements Come From?
The majority of the heavier elements, such as iron, are produced only by the intense chemical and physical reactions within stars.
When supernovas occur, they scatter heavier elements into space, where they eventually are involved in the formation of planets.
The late astronomer and philosopher Carl Sagan frequently referred to humans as “star stuf.”
Atomic Structure
Atomic theory
A theory that states that elements consist of tiny particles called atoms.
Each element consists of only one kind of atom; it is because of this the same name is given to an element and its atoms; This name is represented by one or two letters, called the atomic symbol.
For example, the symbol H stands for an atom of hydrogen, and the symbol Na (for natrium in Latin) stands for an atom of sodium.
A single atom is made mostly of three types of subatomic particles, which include the following:
Neutrons
Contain no electrical charge.
Protons
Contain a positive charge.
Electrons
Contain a negative charge.
Protons and neutrons are located within the center of an atom, which is called the nucleus.

Electrons are in a constant state of motion, so their estimated location is often shown as a cloud.
Most of an atom is empty space.
In an atom, the mass is just about equal to the sum of its protons and neutrons.
Protons and neutrons are assigned one mass unit each.
Electrons, being matter, have mass, but they are so small that their mass is assumed to be zero in most calculations.
The term mass is used rather than weight because mass is constant, but weight is associated with gravity and thus varies depending on an object's location in the universe.
The number of protons makes an atom unique and may be used to identify which element the atom belongs to.
This is called the atom’s atomic number.
All atoms of an element have the same number of protons.
The number of neutrons may vary between atoms of an element.
Atomic mass
The average of the mass numbers of atoms.
The atomic number tells you the number of positively charged protons.
If the atom is electrically neutral, then the atomic number also indicates the number of negatively charged electrons.

Once chemists discovered a number of the elements, they began to realize that the elements’ chemical and physical characteristics recur in a predictable manner.
In a periodic table, the atomic number is written above the atomic symbol, and the atomic mass is written below the atomic symbol.

Noble gases
Gases that rarely react with another atom, such as helium (He) and neon (Ne)
Isotopes
Isotopes
Atoms of the same element that differ in the number of neutrons.
A nucleus with excess neutrons is unstable and may decay and emit radiation; Although
Such an isotope is said to be radioactive
not all isotopes are radioactive.
The radiation given off by radioactive isotopes can be detected in various ways, including a Geiger counter.
Uses of Radioactive Isotopes
The importance of chemistry to biology and medicine is nowhere more evident than in the many uses of radioactive isotopes, including:
As tracers to detect molecular changes or to destroy abnormal or infectious cells.
Positron-emission tomography (PET).
Radioactive substances in the environment can cause harmful chemical changes in cells, damage DNA, and cause cancer.
The release of radioactive particles following a nuclear power plant accident can have far-reaching and long-lasting effects on human health.
But the effects of radiation can also be put to good use, such as in the following examples:
Packets of radioactive isotopes can be placed in the body so that the subatomic particles emitted destroy only cancer cells, with little risk to the rest of the body.
Radiation from radioactive isotopes has been used for many years to sterilize medical and dental equipment.
Mail that is destined for the White House and congressional offices in Washington, DC, is irradiated to protect against dangerous biological agents, such as anthrax.
Arrangement of Electrons in an Atom
The electrons of an atom are constantly moving; Although it is not possible to determine the precise location of an individual electron at any given moment, it is helpful to construct models of atoms that show electrons at discrete energy levels about the nucleus.
The discrete energy levels are known as Electron shells
Electrons orbit the nucleus at particular energy levels called electron shells. The first shell contains up to two electrons, and each shell thereafter has an increasing number of electrons.

Each electron shell contains a certain number of electrons.
Since the nucleus of an atom is positively charged, negatively charged electrons require an increasing amount of energy to push them farther away from the nucleus; It is because of this that Electrons in outer electron shells contain more energy than those in inner electron shells.
If an atom has only one shell, the outer shell is complete when it has two electrons; If an atom has two or more shells, the outer shell is most stable when it has eight electrons; this is called the octet rule.
Atoms with fewer than eight electrons in the outer shell react with other atoms in such a way that each has a complete outer shell after the reaction.
When atoms give up, accept, or share electrons in order to have eight electrons in the outer shell; This is known as the valence shell.
The valence shell determines chemical reactivity.
The size of an atom is also important.
Suppose carbon (C) and silicon (Si) are both in groups of 4. In that case, that means they can bond to achieve 8 electrons, but if you have C with 2 shells and Si with 3 shells, the smaller carbon atom can bond with other carbon atoms to form long-chained molecules, but the silicon atom is unable to bond.
The chemical properties of atoms—that is, the ways they react—are primarily determined by the arrangement of their electrons.
Types of Chemical Bonds
Molecule
A group of atoms bonded together.
Compound
When a molecule contains atoms of more than one element.
Compounds and molecules contain two primary types of chemical bonds, those being the following:
Ionic
Covalent
The type of bond that forms depends on whether two bonded atoms share electrons or whether one has given electrons to the other.
Ionic Bonding
An ionic bond forms when two atoms are held together by the attraction between opposite charges.
Sodium (Na), with only one electron in its third shell, usually gives up an electron; thus,
once it does so, the second shell, with eight electrons, becomes its outer shell.
Chlorine (CI) tends to take on an electron because its outer shell has seven electrons; If chlorine gets one more electron, it has a completed outer shell, so when a sodium atom and a chlorine atom react; an electron is transferred from sodium to chlorine, and both atoms now have eight electrons in their outer shells.
The sodium ion has one more proton than it has electrons; therefore, it has a net charge of +1 (symbolized by Na+).
The chloride ion has one more electron than it has protons; therefore, it has a net charge of –1 (symbolized by Cl–).
This electron transfer causes these atoms to become ions or charged atoms.
Negatively charged ions often have names that end in “ide,” and thus, Cl– is called a chloride ion.
Ionic compounds are often found as salts, solid substances that usually separate and exist as individual ions in water.
An example is sodium chloride (NaCl) or table salt.
Covalent Bonding
A covalent bond results when two atoms share electrons in order to have a completed outer shell.
In a hydrogen atom, the outer shell is complete when it contains two electrons;
If hydrogen is in the presence of a strong electron acceptor, it gives up its electron to become a hydrogen ion (H+).
If this is not possible, hydrogen can share with another atom and thereby have a completed outer shell.
Scientists opt to use structural formulas instead of complete model drawings since a structural formula is simpler.
Example: H--H
The straight line is used to indicate a pair of shared electrons.
A molecular formula omits the lines that indicate bonds and shows the number of atoms involved.
H2.
Sometimes, atoms share more than two electrons to complete their octets.
A double covalent bond occurs when two atoms share two pairs of electrons.

If writing a double covalent bond in structural formula, it would appear as the following example.
O==O
It is also possible for atoms to form triple covalent bonds, as in nitrogen gas (N2), which can be written as the following:
N≡N
Single covalent bonds between atoms are pretty strong, but double and triple bonds are even stronger.
A single atom may form bonds with more than one other atom.

Chemical Formulas and Reactions
Chemical reactions are very important to organisms.
In a chemical reaction, the molecules are often represented by a chemical formula.
Chemical formulas do not indicate the arrangement of these elements; in fact,
they are sometimes several different structures that can be formed based on a chemical formula.
Reactants
Molecules that participate in a reaction.
Products
Molecules are formed by a reaction.
2.2 Water’s Importance to Life
All organisms are 70–90% water; their cells consist of membranous compartments enclosing aqueous solutions.
The structure of a water molecule gives it unique properties, properties that play an essential role in how living organisms function.

The Structure of Water
The electrons shared between two atoms in a covalent bond are not always shared equally.
Atoms differ in their electronegativity—that is, their affinity for electrons in a covalent bond.
Atoms that are more electronegative tend to hold shared electrons more tightly than do those that are less electronegative; this
unequal sharing of electrons causes the bond to become polar.
Polar
Atoms on both sides of the bond are partially charged even though the overall molecule itself bears no net charge.
The polarity of water molecules causes them to be attracted to one another; Positive hydrogen atoms in one molecule are attracted to the negative oxygen atoms in other water molecules.
This attraction is called a hydrogen bond.
Each water molecule can engage in as many as four hydrogen bonds

The covalent bond is much stronger than a hydrogen bond, but the large number of hydrogen bonds in water makes for a strong attractive force.
The properties of water are due to its polarity and its ability to form hydrogen bonds.
Properties of Water
The properties of water that support life are the following:
Solvency
Cohesion
Adhesion
High surface tension
High heat capacity
Varying density
Water is a Solvent
Due to waters polarity and hydrogen-bonding ability, water dissolves a significant number of substances.
Hydrophilic
Molecules that are attracted to water.
Hydrophobic
Nonionized and nonpolar molecules that are not attracted to water.
When a salt such as sodium chloride (NaCl) is put into water, the negative ends of the water molecules are attracted to the sodium ions, and the positive ends of the water molecules are attracted to the chloride ions; This attraction causes the sodium ions and the chloride ions to break up, or dissociate, in water.

Water may also dissolve polar nonionic substances, such as long chains of glucose, by forming hydrogen bonds with them.
When ions and molecules disperse in water, they move about and collide, allowing reactions to occur.
Water Molecules Are Cohesive and Adhesive
Cohesion
The ability of water molecules to cling to each other due to hydrogen bonding.
Because of cohesion, water exists as a liquid under ordinary conditions of temperature and pressure.
Adhesion
The ability of water molecules to cling to other polar surfaces.
Adhesion is due to water’s polarity; the positive and negative poles of water molecules cause them to adhere to other polar surfaces.
Due to cohesion and adhesion, liquid water is an excellent transport system.
Both within and outside the cell, water assists in the transport of nutrients and waste materials.
The liquid portion of blood, which transports dissolved and suspended substances within the body, is 90% water.
The cohesion and adhesion of water molecules allow blood to fill the tubular vessels of the cardiovascular system, making transport possible.
Cohesion and adhesion also contribute to the transport of water in plants.
Water Has a High Surface Tension
Because the water molecules at the surface are more strongly attracted to each other than to the air above, water molecules at the surface cling tightly to each other, and that is why we say that water exhibits surface tension.
The stronger the force between molecules in a liquid, the greater the surface tension.
Hydrogen bonding is the main force that causes water to have high surface tension.
Water Has A High Heat Capacity
The many hydrogen bonds that link water molecules allow water to absorb heat without greatly changing in temperature.
Water’s high heat capacity is essential for all organisms
Because the temperature of water rises and falls slowly, terrestrial organisms are better able to maintain their normal internal temperatures and are protected from rapid temperature changes.
Water also has a high heat of vaporization: It takes a great deal of heat to break the hydrogen bonds in water so that it becomes gaseous and evaporates into the environment.
Because of water’s high heat capacity and high heat of vaporization, temperatures along the majority of Earth’s coasts are moderate.
Ice is Less Dense Than Water
Unlike other substances, water expands as it freezes; Since water expands as it freezes, ice is less dense than liquid water, and therefore ice floats on liquid water.
2.3 Acids and Bases
Acidic Solutions (High H+ Concentration)
Acidic solutions have a sharp or sour taste, and therefore we sometimes associate them with indigestion.
Acids
Substances that dissociate in water release hydrogen ions (H+).
Acidic solutions have a higher concentration of H+ ions than OH– ions.
The acidity of a substance depends on how fully it dissociates in water.
HCl dissociates almost completely; therefore, it is called a strong acid.
Basic Solutions (Low H+ Concentration)
Milk of magnesia and ammonia are common basic (alkaline) solutions that most people are familiar with.
Basic solutions have a bitter taste and feel slippery when in water.
Bases
substances that either take up hydrogen ions (H+ ) or release hydroxide ions (OH– ).
For example, an important base is sodium hydroxide (NaOH), which dissociates in this manner.
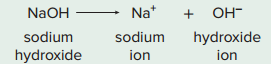
Basic solutions have a higher concentration of OH– ions than H+ ions. Like acids, the strength of a base is determined by how fully it dissociates; the dissociation of sodium hydroxide is almost complete; therefore, it is called a strong base.
Many strong bases, such as ammonia, are useful household cleansers.
pH and the pH Scale
pH1 is a mathematical way of indicating the number of hydrogen ions in a solution.
The pH scale is used to indicate the acidity or basicity of a solution.
The pH scale ranges from 0 to 14, where 7 represents neutrality, below 7 equals acidic due to the hydrogen ion concentration, and above 7 is basic because [OH–] is greater than [H+ ].
Moving down the pH scale from pH 7 to pH 0, each unit has 10 times the acidity [H+ ] of the previous unit, similarly As we move up the scale from 7 to 14, each unit has 10 times the basicity [OH– ] of the previous unit.
The pH scale was devised to eliminate the use of cumbersome numbers.
The effect of pH on organisms is dramatically illustrated by the phenomenon known as acid precipitation.

Buffers and pH
Buffer
A chemical or a combination of chemicals that keeps pH within established limits.
Buffers resist pH changes because they can take up excess hydrogen ions (H+ ) or hydroxide ions (OH– ).
In the human body, pH needs to be kept within a narrow range in order to maintain homeostasis.
Diseases such as diabetes and congestive heart failure may bring on a condition called acidosis, in which the body is unable to buffer the excessive production of H+ ions.
If left untreated, acidosis can cause a number of health problems and may result in coma or death.
 Knowt
Knowt
