
Saponification
the reaction of an ester with sodium hydroxide will hydrolyze the ester into a carboxylate salt and an alcohol
this is a superior method since the formation of a carboxylate makes this reaction irreversible
this is the general reaction:

this reaction is irreversible because carboxylate salts are more stable than carboxylic acids and thus do not react under the same conditions
this is a special case of LeChatelier’s Principle since one of the products is removed
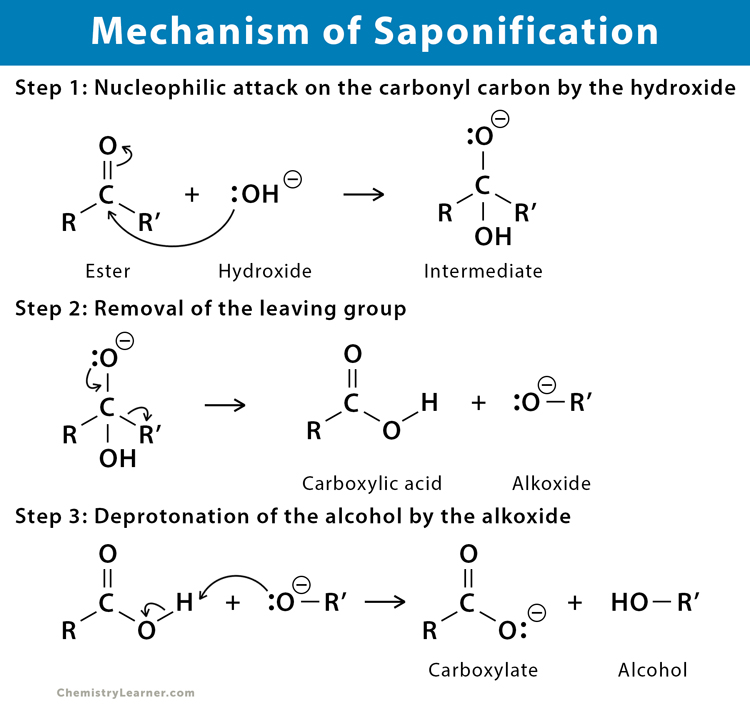
mechanism of saponification:
“Saponification” itself means “making soap”
it comes from the reaction of fats with bases to form soap
Fats and oils are triglycerides/triacylglycerols: triesters of glycerol
when the triglycerides are hydrolyzed, sodium salts of the fatty acids are formed
these make soap an effective surfactant
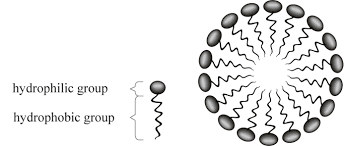
they are amphipathic: having both polar and nonpolar regions
soap acts as a cleaning agent because it forms micelles: spherical structures formed by the self-assembly of amphipathic molecules
these micelles can trap nonpolar dirt inside the nonpolar portions of the fatty acid salts
Saponification
the reaction of an ester with sodium hydroxide will hydrolyze the ester into a carboxylate salt and an alcohol
this is a superior method since the formation of a carboxylate makes this reaction irreversible
this is the general reaction:

this reaction is irreversible because carboxylate salts are more stable than carboxylic acids and thus do not react under the same conditions
this is a special case of LeChatelier’s Principle since one of the products is removed
mechanism of saponification:

“Saponification” itself means “making soap”
it comes from the reaction of fats with bases to form soap
Fats and oils are triglycerides/triacylglycerols: triesters of glycerol
when the triglycerides are hydrolyzed, sodium salts of the fatty acids are formed
these make soap an effective surfactant
they are amphipathic: having both polar and nonpolar regions
soap acts as a cleaning agent because it forms micelles: spherical structures formed by the self-assembly of amphipathic molecules
these micelles can trap nonpolar dirt inside the nonpolar portions of the fatty acid salts

 Knowt
Knowt