
Thermochemistry (in depth)
Energy and Rates
Thermodynamics
The study of energy and energy transfer
Thermochemistry
A branch of chemistry which studies energy changes that accompany physical, chemical and nuclear changes.
Energy (E)
The ability to do work. Work is done when an applied force causes an object to move through a distance in the same direction as the applied forces. Measured in J, KJ, MJ.
Law of conversion of energy
Energy can neither be created nor destroyed, it can be transferred from one substance too another. Or it can be converted from one form to another.
First Law of Thermodynamics
The energy of the universe is constant. ∆E universe= 0.
∆ = change
Kinetic Energy (Ek)
Energy possessed by objects in motion.
Ek= 1/2 mv^2
Potential Energy (Ep)
Stored energy is energy that can be released or harnessed to do work. Energy that a body possesses by virtue of it position.
Remember: Kinetic and potential energy switch back and forth.
Eg. Water held by a dam possess Ep. Bonds holding carbon together in coal possess Ep.
Thermal Energy
Total Ek available in a substance as a result of the motion of its particles. It depends on the total number of particles. The higher number of particles → the greater the thermal energy is.
Heat (q)
Thermal energy that is spontaneously transferred from a warmer object to a cooler object.
Temperature
The average Ek of particles in a substance.
Chemical System
Reactants and products under study as represented in the chemical equation. Energy is either released or absorbed as the reactants are transformed into the product.
Surroundings
Matter surrounding the system which is capable of absorbing or releasing thermal energy.
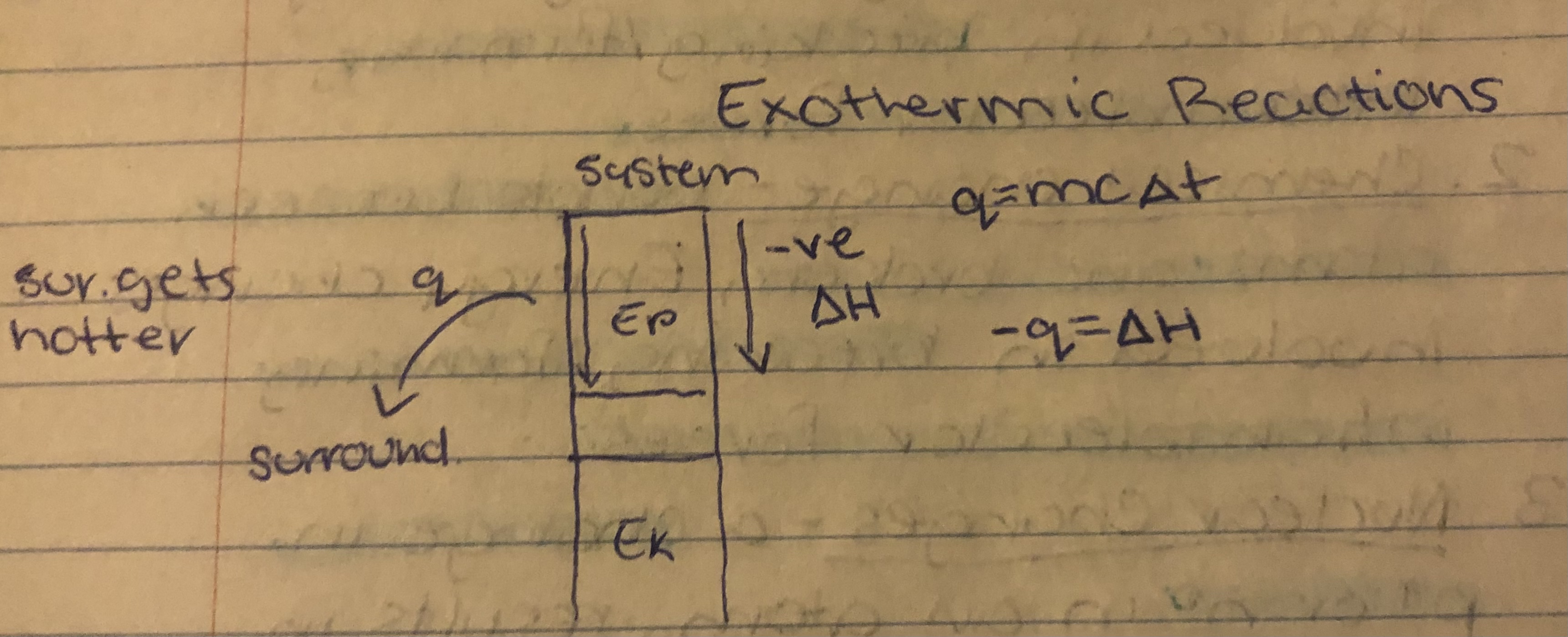
Exothermic
System transfers heat to surroundings, temp of surroundings increases.
Eg. Combustion, freezing, condensation, nuclear fission and fusion.
Endothermic
System absorbs heat from surroundings, temp of surroundings decreases.
Eg. Melting, vaporizing, breaking chemical bonds
Isolated systems
An ideal system in which neither matter nor energy can move in or out.
Eg. Styrofoam cups, thermos.
Open System
Matter and energy can move in or out of the system.
Eg. Beaker with a chemical reaction occurring.
Closed system
Only energy can move in and out, matter cannot.
Eg. Beaker with lid with chemical reaction occurring inside.
Calorimetry
Technological process of measuring energy changes in a chemical system.
Calorimeter
A calorimeter works by insulating a system from its surroundings. The amount of energy/heat that can be transferred into of out of a substance to facilitate a temperature change.
It depends on 3 factors.
Mass (m) [Kg, g]
The larger the mass, the more energy required to increase the tempurature.
Specific heat Capacity (c) [J/g℃, J/g^K)
Amount of energy required to increase the temperate of 1g of a substance by 1℃. The higher the value of c is, the more energy is required to increase 1g by 1℃.
Temperature change (∆t) [C or K]
The greater the desired temperature change is, the more heat must be transferred into or out of the substance.
∆T= t(final)- t(initial)
Q= m c ∆t
Q→ heat(J)
M→ mass (g)
C→ specific heat capacity (J/g℃)
∆T→ change in tempurature (℃)
If the tempurature gets hotter the ∆t=+ve. Because energy is gained.
If the tempurature gets cooler ∆t=-ve.. Because energy is lost.
Enthalpy
The total Ek and Ep of a chemical system.
Forms of Ek in chemical system
Vibrations of atoms held together by bonds. Rotation and translation of molecules.
Forms of Ep in chemical systems
Electrostatic Ep of atoms connected by chemical bonds.
Change in Enthalpy(∆H)
The difference in enthalpy between reactants and products during physical or chemical changes. It is the measuring of energy absorbed or released by the surroundings.
Exothermic Reactions
 Endothermic Reactions
Endothermic Reactions
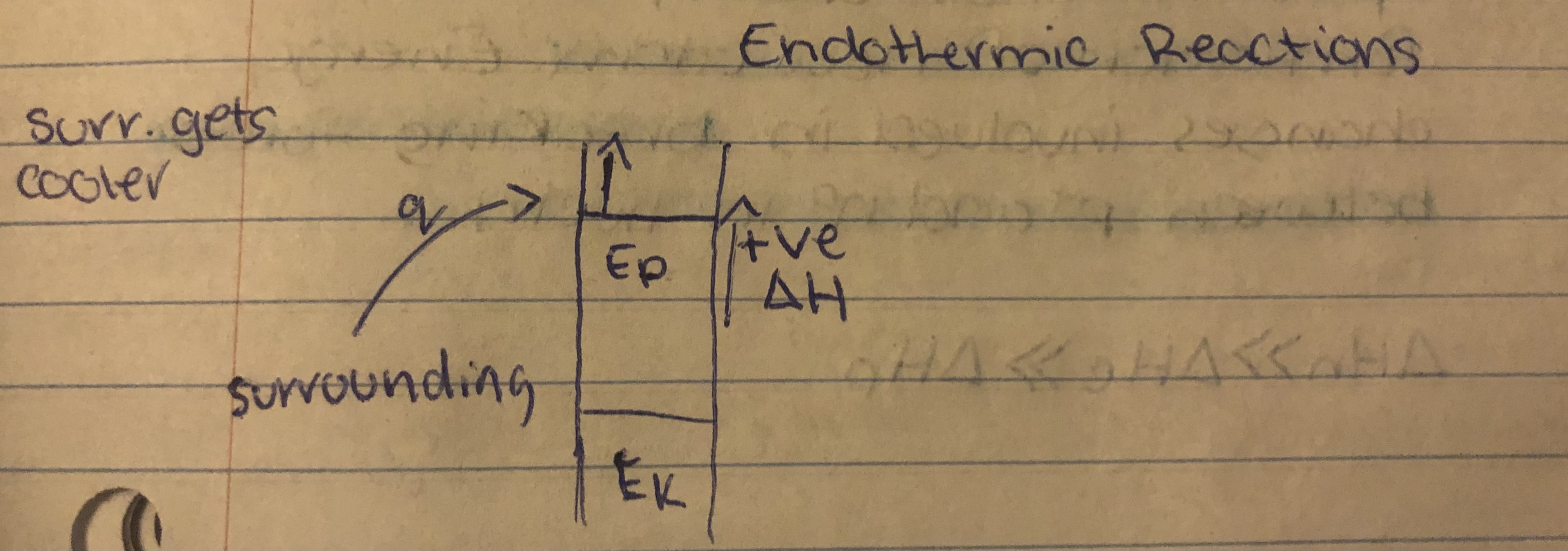
In an endothermic change…
The Ep stored in product bonds > Ep stored in the reactant bonds. Enthalpy of system increases. ∆H<0.
In an exothermic change…
The Ep stored in the product bonds < Ep stored in the reactant bonds.
Types of enthalpy changes.
Physical Change
Energy changes involved in breaking/forming intermolecular forces.
Chemical Change
Bonds between atoms are broken. Energy changes involved in breaking/forming Intramolecular forces.
Nuclear Changes
A change in p+ or n0 in an atom. Results in formation of new atom. Energy changes involved in breaking forces between p+ and n0 in the nucleus.
∆Hn>>∆Hc>>∆Hp
Calculating ∆H
∆H can be found for a reaction in several ways.
Molar enthalpy
Calorimetry
Bond energy
Hess’s law
∆Hf (formation)
Molar Enthalpy (∆Hx)
Enthalpy change associated when 1 mol of a substance undergoes a physical, chemical or nuclear change.
Reminder: mass →mm→mol. Vol→mv→mol
∆H= n∆Hx
∆H→ total enthalpy change (KJ, J)
N→ mol
∆Hx→ molar enthalpy (KJ/mol, J/mol)
Determining ∆H and ∆Hx for physical and chemical changes from experiment.
Based on the following principle
∆H= -q <— total enthalpy
N∆Hx= -mc∆t <— molar enthalpy
Assumptions
No heat is transferred between calorimeter and surroundings.
Any heat exchange with calorimeter materials is negligible.
A dilute aqueous solution is assumed to have the same density(1.00m/ml) and same specific heat capacity (4.18J/g℃) as H2O.
Thermochemical Equations
A balanced chemical equation that indicates the amount of energy that is absorbed or released by the reaction it represents. Can be represented in 4 different ways.
Examples for this lesson.
Photosynthesis (endothermic) = 6CO2+6H2O→C6H12O6+6O2
Cellular Respiration (exothermic)= C6H12O6+6O2→6CO2+6H2O
Energy term is part of the chemical equation.
Photosynthesis= 6CO2+6H2O+2802.7KJ→C6H12O6+6O2
Cellular Respiration= C6H12O6+6O2→6CO2+6H2O+2802.7KJ
∆H terms
+ve → endothermic. -ve→ exothermic.
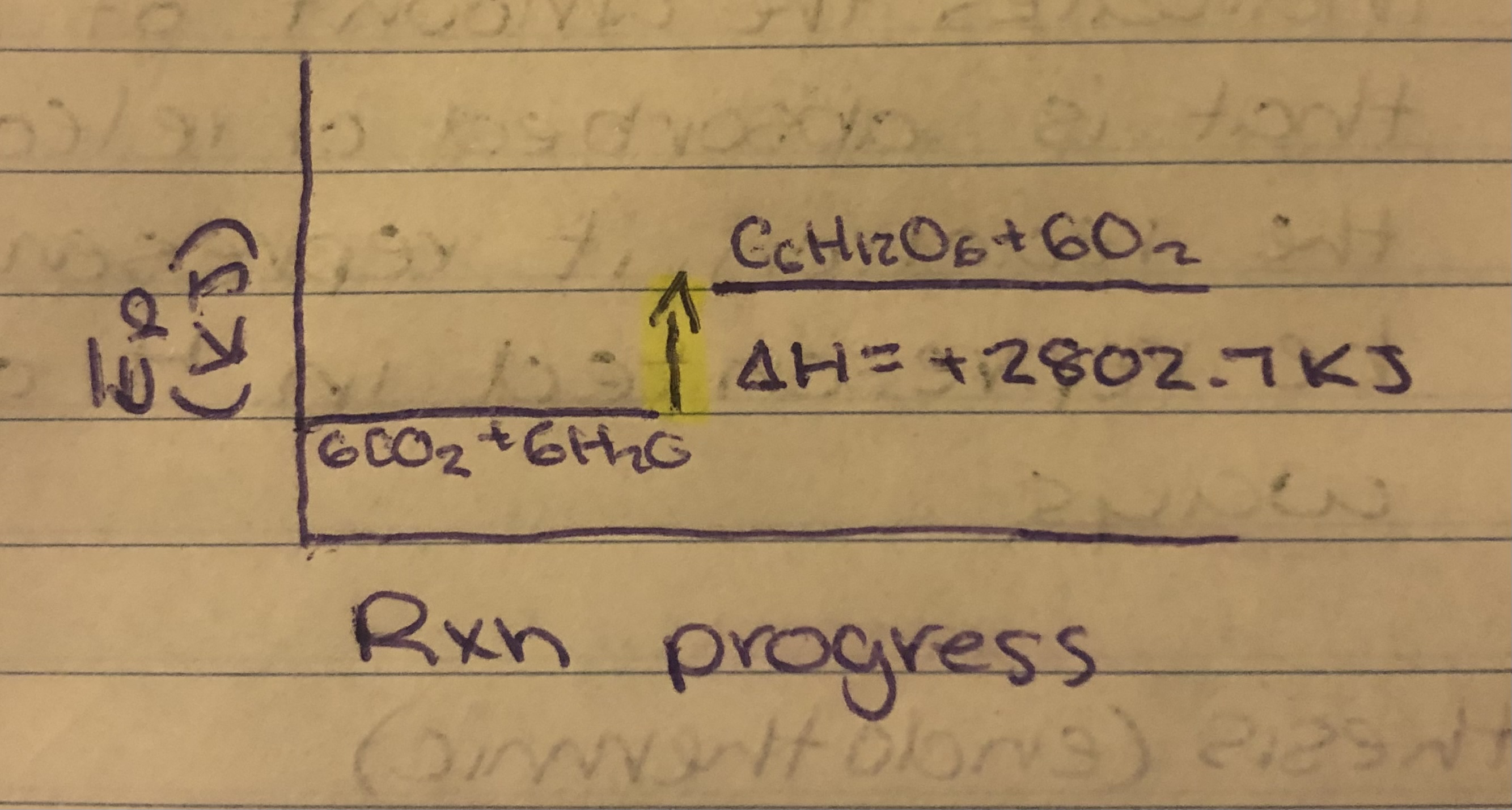
Photosynthesis= 6CO2+6H2O→C6H12O6+6O2. ∆H=2802.7KJ
Cellular Respiration= C6H12O6+6O2→6CO2+6H2O. ∆H=-2802.7KJ
As molar enthalpy of reaction
6CO2+6H2O→C6H12O6+6O2 ∆H=2802.7KJ
∆HPhotosynthesis= 2802.7KJ/1mol C6H12O6
Use C6H12O6 because it has a coefficient on 1. Same rule with cellular respiration.
Ep diagram
Photosynthesis
Cellular Respiration
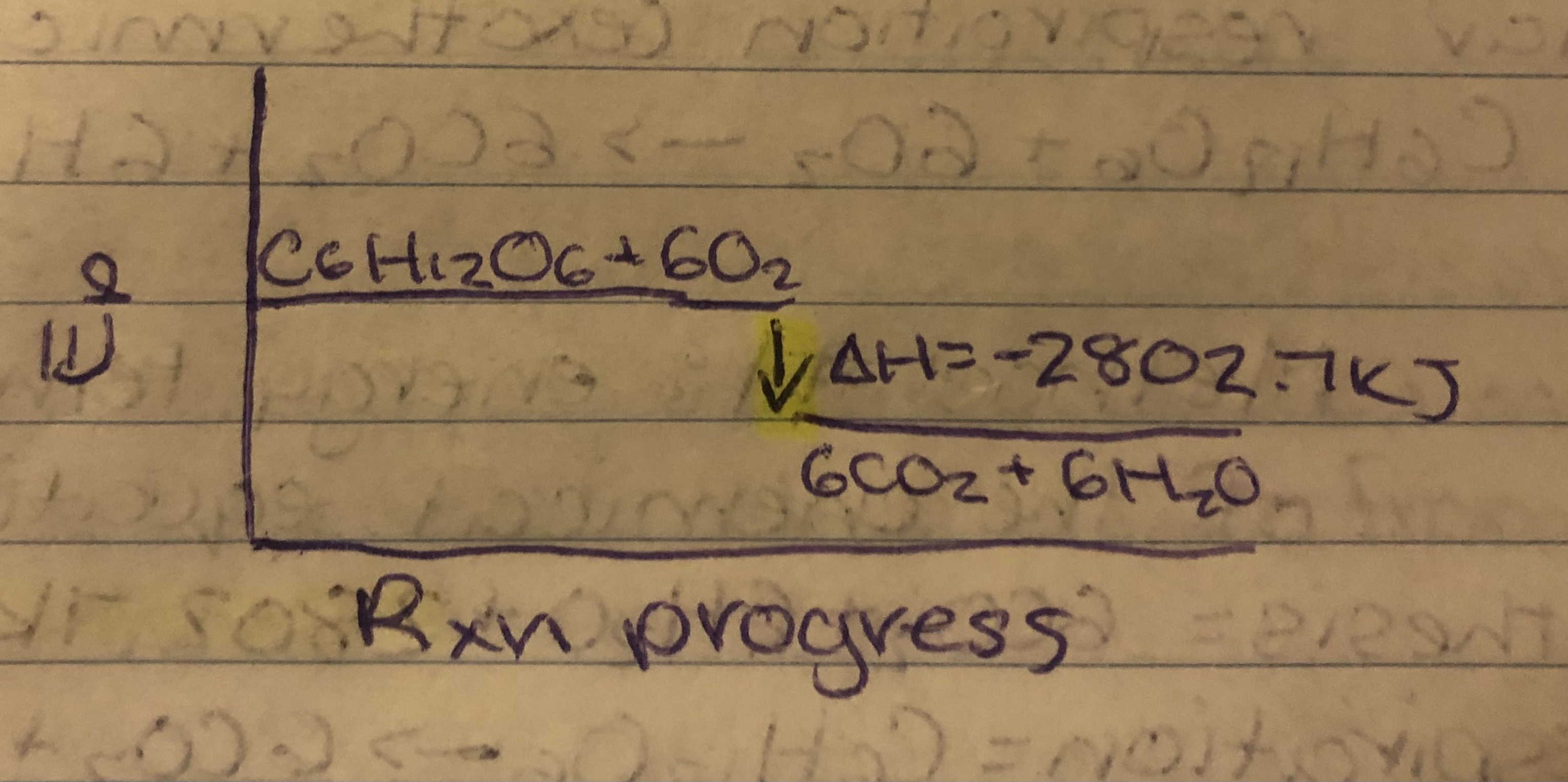
∆H From Bond Energies
Reactants
Bonds broken
Energy is needed
Endothermic process
Products
Bonds formed
Energy Released
Exothermic process
Bond Energy- the amount of energy required to break one mole of bonds between 2 particular atoms (endothermic). The amount of energy released when one mole of bonds is formed between 2 particular atoms (exothermic).
Calculating ∆H Using Bond Energies.
∆H= Σ Reactant bond energies - Σ Product bond energies
= (Σn x D bond broke)-(Σn x D bonds formed)
Reminder: Σ (sigma) means ‘the sum of’. D- Bond energy
Hess’s Law of Additivity of Reaction Enthalpy
Many chemical reactions that don’t occur in aqueous solutions or that release so much energy that it is not appropriate to preform them in a coffee cup calorimeter. Usually reactions can be broken down into series of simpler reactions, when combined have they have the same initial reactants and final products a original reactions.
∆H For these reactions can be determined by using Hess’s law.
Hess’s Law
∆H For any reactions that can be written in steps equals the sum of the values of ∆H for each of the individual steps.
∆HTarget= ∆H1+∆H2+…
Rules for Hess’s Law
If a chemical equation is reversed then the sign of ∆H changes.
If the coefficient of a chemical equation are altered by multiplying or dividing by a constant factor then ∆H is altered in the same way.
Using Standard Heats of Formation to Get Enthalpy
Standard Enthalpy of formation (ΔHf)
The quantity of energy is absorbed/released when 1mol of substance is formed directly from its elements in their standard states (SATP). ΔHf are tabulated enthalpy changes for a special set of reactions called formation reactions.
ΔH= ΣnΔHf products - ΣnΔHf reactants
Entropy and Disorder
Entropy (s)
A measure of disorder of a microscopic system
Change in Entropy (ΔS)
ΔS is the difference of enthalpy between reactants and products. It can be +ve or -ve.
+ve = order→disorder
-ve = disorder→order
ΔS=0 means order is perfect.
Eg. Increasing Entropy
In chemical reactions entropy increases when:
Fewer moles of reactants produce greater number of moles of product in the same state
Complies molecules broken into simpler substances
Solid reactants become liquid or gas (or when liquid becomes gas).
ΔS= ΣnS products - ΣnS reactants
Thermodynamics and Spontaneous Reactions
Spontaneous Reactions
Occurs without continuous outside interferences under a given set of conditions. Reactants become products.
A+B=C
Non-spontaneous Reactions
Doesn’t occur without continuous outside interference. Reactants do not become products.
A+B=/= C
Law of Enthalpy
Reactants go from high Ep to low Ep as spontaneous with respect to energy. This results in transfer of energy as heat from system to surroundings (exothermic).
ΔH<0 or ΔH=-ve
Chemical systems react to minimize the enthalpy.
A reaction is spontaneous if enthalpy of system decreases. And a reaction is non-spontaneous if the enthalpy of the system increases.
Law of Entropy
A reaction is spontaneous if entropy of system increases. If a reaction is non-spontaneous the entropy decreases.
Recap
A reaction will always be spontaneous if (ΔH<0) and (ΔS>0).
A reaction will always be non-spontaneous if (ΔH>0) and (ΔS<0).
Reactions will be reversible if ΔH and ΔS have the same sign.
Gibbs Energy
Gibbs Free Energy
Mathematical combination of ΔH and ΔS. It is used to determine spontaneity of chemical reactions. Determines total useful energy available.
ΔG= ΔH-TΔS
Criteria
ΔG= -ve, the reaction is spontaneous and does useful work for the surroundings.
ΔG= +ve, reaction is non-spontaneous requires work from surroundings.
ΔG= 0, the reaction is at equilibrium.
Temperature Effect on ΔG
Low tempurature term is small, look at ΔH to determine if spontaneous.
High tempurature term is large, look at ΔS to determine if spontaneous.
If ΔH(-ve) and ΔS(+ve) are both spontaneous, then the reaction will be spontaneous no matter the temperature. (Opposite for non-spontaneous).
Introduction to Rates of Reactions
Chemical Kinetics- the study of the rates of chemical reactions
Reaction rate- the speed in which a chemical reaction occurs. It is determined by measuring
The rate at which a reactant is consumed.
The rate at which a product is produced.
Average and Instantaneous Reaction Rates
reaction rates are not constant, they vary with time
As reaction proceeds, the reactants decrease
As reaction proceeds, product increases
Average Rate of Reaction
Change in reactant or product over a given time interval. Can be determined by:
Using a data table
Determining the slope of a secant
Instantaneous Rate of Reaction
rate of reaction at a particular point in time
Determine by measuring the slope of a tangent
Reminder: Slope= rise/run
Measuring Reaction Rates
There are many properties that chemists can measure to find reaction rates. The method used depends on the nature of substance and the type or reaction. Measurements must be made without disturbing the progress of the reaction.
Change in Colour
some substances change colour as they produce new substances
Change in pH
if the H+ or OH- changes in the reactions, the pH will change.
Change in Electrical Conductivity
total number of ions changes from reactants to products. The ability of the system to conduct electricity is affected.
Changes in Volume or Pressure of Gas
if a gas is produced in a reaction. The change in volume/pressure over a given time period can be used to determine the rate.
Rate Laws and Order of Reaction
[ ] = concentration
Rate Law
rate of reaction [reactant]. As [reactant] increases, so does rate.
Rate law is the relationship between initial rate of reaction and initial concentration of reactant.
A balanced chemical equation is needed.
R=K[A]^x[B]^y
Rate Constant (k):
k is constant for any concentration of reactant at a given temp
The value of k varies with temp and is determined experimentally
Rate Law exponents (x and y)
do not vary with temp And are determined experimentally
Describe dépendance of reaction rate of initial concentration
Also called individual orders of reaction
Collision Theory
Factors that affect reaction rates:
Chemical Nature of Reactants
metals lower on the activity series are less reactive.
Reactions of monoatomic ions in aqueous solutions are very fast.
Reactions of molecular substances are often slow due to bonds needing to be broken and formed in the process.
Concentration
increasing initial concentration of reactants generally increases the reaction rates.
Temperature
increasing temp increases the reaction rate.
Rule of thumb: a 10C increase in temp doubles the reaction rate.
Catalyst
increases the reaction rate without being consumed.
Surface Area
increasing the surface area of solid reactants increases the reaction rate.
Collision Theory and Rates of Reaction
for a chemical reaction to occur, the reacting particles must collide with each other. But not every collision is effective. Not every collision leads to a reaction.
Conditions For An Effective Collision
Particles must collide with proper collision geometry.
Particles must collide with enough energy to break bonds in reacting particles.
Threshold Energy (Et)
minimum amount of Ek with which the reacting particles must collide to result in a reaction.
Activation Energy (Ea)
minimum amount of Ep that is required before the reactants can rearrange in structure and thus react.
An Effective Collision:
reacting particles collide with the proper collision geometry and sufficient energy so that bonds can break and new bonds can form.
An Ineffective Collision:
reacting particles rebound unchanged from the collision due to improper geometry and energy.
Collision Energy
depends on Ek of colliding particles
The higher the temp, the higher the Ek
Temp is the measure of average Ek
Maxwell-Boltzmann distribution curve- shows the relationship between the number of particles vs. Ek at a given temp. At a higher temp more particles have enough energy to overcome Et barrier.
Therefore reaction rate depends on:
Frequency of collision
Fraction of effective collisions
Rate= frequency x fraction
Concentration
increase of concentration→increases # of reacting particles
Increases frequency of collision and rate
Surface Area
Increase S.A→increases # of collisions
Increases frequency and rate
Temperature
Increase temp→increase spreed of particles
Increases frequency and fraction of effectiveness. Increases rate.
Nature of Reactants
effects fraction of effective collisions
Molecules with weak bonds have lower Ea, require less Ek to collide
Complicated molecules or ions require more Ek and less likely to collide in correct orientation.
Catalyst
provides alternative pathway with lower Ea→larger # of reactants have enough Ek to react.
# of effective collisions and rate increases.
Transistion State Theory
Used to explain what happens after molecules collide. And examines what happens as reactants change to products.
As reactants approach each other:
repulsive forces cause reactants to slow down
Ek converted to Ep
Et=Ea
Bond structure rearranges to form the product, only if enough Ep is stored.
As new molecules form, repulsive forces push molecules apart. Increase Ek.
Ea: difference between energy of reactants and the peak of the Ep curve.
H- difference between energy in reactants and products.
Activated Complex- unstable chemical species that contains partially broken and partially formed bonds. It represents the max Ep point. It’s formed only if reactants collide with enough energy and is formed during transition state.
Reaction Mechanisms
Thermochemistry (in depth)
Energy and Rates
Thermodynamics
The study of energy and energy transfer
Thermochemistry
A branch of chemistry which studies energy changes that accompany physical, chemical and nuclear changes.
Energy (E)
The ability to do work. Work is done when an applied force causes an object to move through a distance in the same direction as the applied forces. Measured in J, KJ, MJ.
Law of conversion of energy
Energy can neither be created nor destroyed, it can be transferred from one substance too another. Or it can be converted from one form to another.
First Law of Thermodynamics
The energy of the universe is constant. ∆E universe= 0.
∆ = change
Kinetic Energy (Ek)
Energy possessed by objects in motion.
Ek= 1/2 mv^2
Potential Energy (Ep)
Stored energy is energy that can be released or harnessed to do work. Energy that a body possesses by virtue of it position.
Remember: Kinetic and potential energy switch back and forth.
Eg. Water held by a dam possess Ep. Bonds holding carbon together in coal possess Ep.
Thermal Energy
Total Ek available in a substance as a result of the motion of its particles. It depends on the total number of particles. The higher number of particles → the greater the thermal energy is.
Heat (q)
Thermal energy that is spontaneously transferred from a warmer object to a cooler object.
Temperature
The average Ek of particles in a substance.
Chemical System
Reactants and products under study as represented in the chemical equation. Energy is either released or absorbed as the reactants are transformed into the product.
Surroundings
Matter surrounding the system which is capable of absorbing or releasing thermal energy.
Exothermic
System transfers heat to surroundings, temp of surroundings increases.
Eg. Combustion, freezing, condensation, nuclear fission and fusion.
Endothermic
System absorbs heat from surroundings, temp of surroundings decreases.
Eg. Melting, vaporizing, breaking chemical bonds
Isolated systems
An ideal system in which neither matter nor energy can move in or out.
Eg. Styrofoam cups, thermos.
Open System
Matter and energy can move in or out of the system.
Eg. Beaker with a chemical reaction occurring.
Closed system
Only energy can move in and out, matter cannot.
Eg. Beaker with lid with chemical reaction occurring inside.
Calorimetry
Technological process of measuring energy changes in a chemical system.
Calorimeter
A calorimeter works by insulating a system from its surroundings. The amount of energy/heat that can be transferred into of out of a substance to facilitate a temperature change.
It depends on 3 factors.
Mass (m) [Kg, g]
The larger the mass, the more energy required to increase the tempurature.
Specific heat Capacity (c) [J/g℃, J/g^K)
Amount of energy required to increase the temperate of 1g of a substance by 1℃. The higher the value of c is, the more energy is required to increase 1g by 1℃.
Temperature change (∆t) [C or K]
The greater the desired temperature change is, the more heat must be transferred into or out of the substance.
∆T= t(final)- t(initial)
Q= m c ∆t
Q→ heat(J)
M→ mass (g)
C→ specific heat capacity (J/g℃)
∆T→ change in tempurature (℃)
If the tempurature gets hotter the ∆t=+ve. Because energy is gained.
If the tempurature gets cooler ∆t=-ve.. Because energy is lost.
Enthalpy
The total Ek and Ep of a chemical system.
Forms of Ek in chemical system
Vibrations of atoms held together by bonds. Rotation and translation of molecules.
Forms of Ep in chemical systems
Electrostatic Ep of atoms connected by chemical bonds.
Change in Enthalpy(∆H)
The difference in enthalpy between reactants and products during physical or chemical changes. It is the measuring of energy absorbed or released by the surroundings.
Exothermic Reactions
 Endothermic Reactions
Endothermic Reactions

In an endothermic change…
The Ep stored in product bonds > Ep stored in the reactant bonds. Enthalpy of system increases. ∆H<0.
In an exothermic change…
The Ep stored in the product bonds < Ep stored in the reactant bonds.
Types of enthalpy changes.
Physical Change
Energy changes involved in breaking/forming intermolecular forces.
Chemical Change
Bonds between atoms are broken. Energy changes involved in breaking/forming Intramolecular forces.
Nuclear Changes
A change in p+ or n0 in an atom. Results in formation of new atom. Energy changes involved in breaking forces between p+ and n0 in the nucleus.
∆Hn>>∆Hc>>∆Hp
Calculating ∆H
∆H can be found for a reaction in several ways.
Molar enthalpy
Calorimetry
Bond energy
Hess’s law
∆Hf (formation)
Molar Enthalpy (∆Hx)
Enthalpy change associated when 1 mol of a substance undergoes a physical, chemical or nuclear change.
Reminder: mass →mm→mol. Vol→mv→mol
∆H= n∆Hx
∆H→ total enthalpy change (KJ, J)
N→ mol
∆Hx→ molar enthalpy (KJ/mol, J/mol)
Determining ∆H and ∆Hx for physical and chemical changes from experiment.
Based on the following principle
∆H= -q <— total enthalpy
N∆Hx= -mc∆t <— molar enthalpy
Assumptions
No heat is transferred between calorimeter and surroundings.
Any heat exchange with calorimeter materials is negligible.
A dilute aqueous solution is assumed to have the same density(1.00m/ml) and same specific heat capacity (4.18J/g℃) as H2O.
Thermochemical Equations
A balanced chemical equation that indicates the amount of energy that is absorbed or released by the reaction it represents. Can be represented in 4 different ways.
Examples for this lesson.
Photosynthesis (endothermic) = 6CO2+6H2O→C6H12O6+6O2
Cellular Respiration (exothermic)= C6H12O6+6O2→6CO2+6H2O
Energy term is part of the chemical equation.
Photosynthesis= 6CO2+6H2O+2802.7KJ→C6H12O6+6O2
Cellular Respiration= C6H12O6+6O2→6CO2+6H2O+2802.7KJ
∆H terms
+ve → endothermic. -ve→ exothermic.
Photosynthesis= 6CO2+6H2O→C6H12O6+6O2. ∆H=2802.7KJ
Cellular Respiration= C6H12O6+6O2→6CO2+6H2O. ∆H=-2802.7KJ
As molar enthalpy of reaction
6CO2+6H2O→C6H12O6+6O2 ∆H=2802.7KJ
∆HPhotosynthesis= 2802.7KJ/1mol C6H12O6
Use C6H12O6 because it has a coefficient on 1. Same rule with cellular respiration.
Ep diagram
Photosynthesis

Cellular Respiration
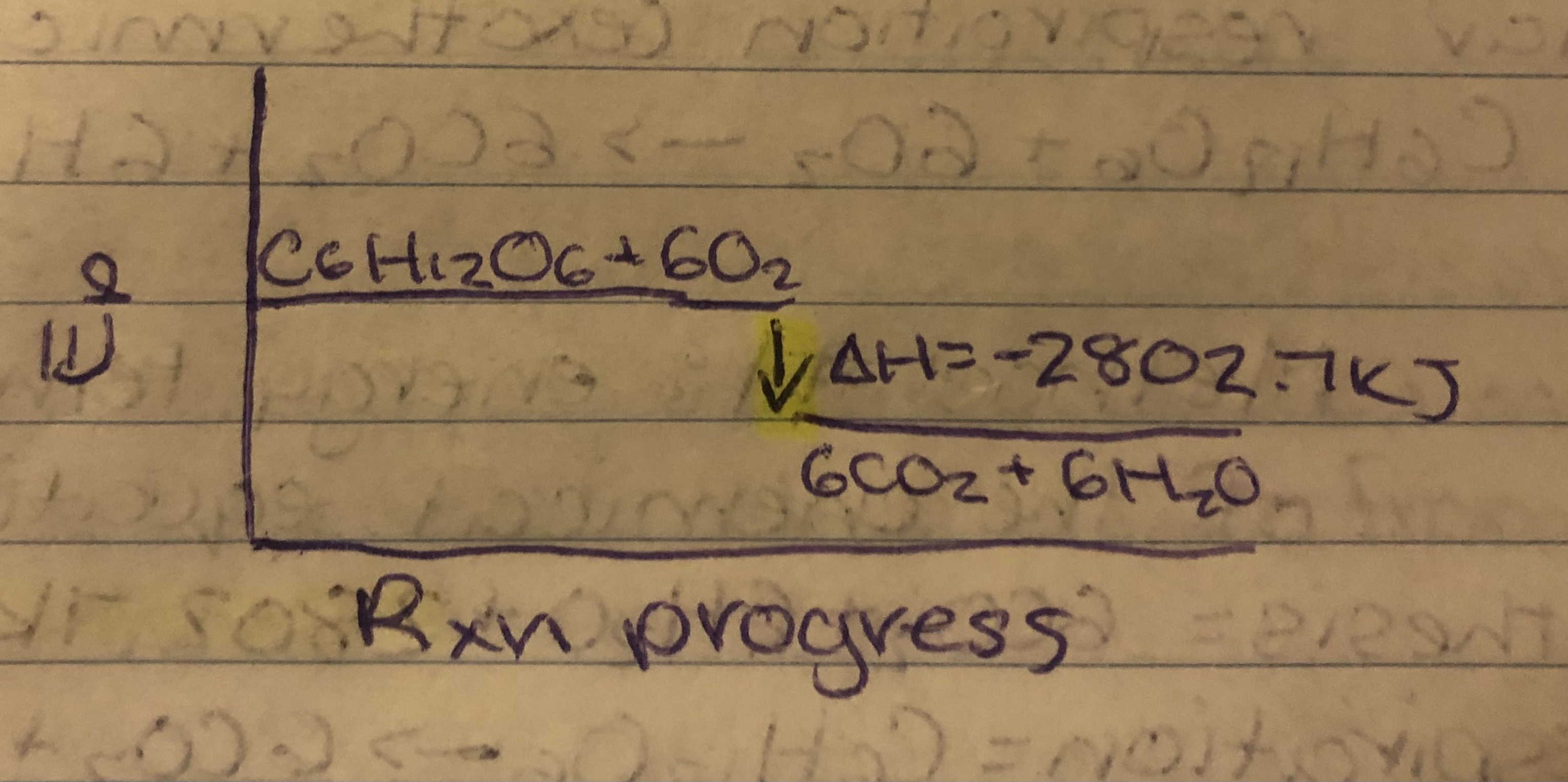
∆H From Bond Energies
Reactants
Bonds broken
Energy is needed
Endothermic process
Products
Bonds formed
Energy Released
Exothermic process
Bond Energy- the amount of energy required to break one mole of bonds between 2 particular atoms (endothermic). The amount of energy released when one mole of bonds is formed between 2 particular atoms (exothermic).
Calculating ∆H Using Bond Energies.
∆H= Σ Reactant bond energies - Σ Product bond energies
= (Σn x D bond broke)-(Σn x D bonds formed)
Reminder: Σ (sigma) means ‘the sum of’. D- Bond energy
Hess’s Law of Additivity of Reaction Enthalpy
Many chemical reactions that don’t occur in aqueous solutions or that release so much energy that it is not appropriate to preform them in a coffee cup calorimeter. Usually reactions can be broken down into series of simpler reactions, when combined have they have the same initial reactants and final products a original reactions.
∆H For these reactions can be determined by using Hess’s law.
Hess’s Law
∆H For any reactions that can be written in steps equals the sum of the values of ∆H for each of the individual steps.
∆HTarget= ∆H1+∆H2+…
Rules for Hess’s Law
If a chemical equation is reversed then the sign of ∆H changes.
If the coefficient of a chemical equation are altered by multiplying or dividing by a constant factor then ∆H is altered in the same way.
Using Standard Heats of Formation to Get Enthalpy
Standard Enthalpy of formation (ΔHf)
The quantity of energy is absorbed/released when 1mol of substance is formed directly from its elements in their standard states (SATP). ΔHf are tabulated enthalpy changes for a special set of reactions called formation reactions.
ΔH= ΣnΔHf products - ΣnΔHf reactants
Entropy and Disorder
Entropy (s)
A measure of disorder of a microscopic system
Change in Entropy (ΔS)
ΔS is the difference of enthalpy between reactants and products. It can be +ve or -ve.
+ve = order→disorder
-ve = disorder→order
ΔS=0 means order is perfect.
Eg. Increasing Entropy
In chemical reactions entropy increases when:
Fewer moles of reactants produce greater number of moles of product in the same state
Complies molecules broken into simpler substances
Solid reactants become liquid or gas (or when liquid becomes gas).
ΔS= ΣnS products - ΣnS reactants
Thermodynamics and Spontaneous Reactions
Spontaneous Reactions
Occurs without continuous outside interferences under a given set of conditions. Reactants become products.
A+B=C
Non-spontaneous Reactions
Doesn’t occur without continuous outside interference. Reactants do not become products.
A+B=/= C
Law of Enthalpy
Reactants go from high Ep to low Ep as spontaneous with respect to energy. This results in transfer of energy as heat from system to surroundings (exothermic).
ΔH<0 or ΔH=-ve
Chemical systems react to minimize the enthalpy.
A reaction is spontaneous if enthalpy of system decreases. And a reaction is non-spontaneous if the enthalpy of the system increases.
Law of Entropy
A reaction is spontaneous if entropy of system increases. If a reaction is non-spontaneous the entropy decreases.
Recap
A reaction will always be spontaneous if (ΔH<0) and (ΔS>0).
A reaction will always be non-spontaneous if (ΔH>0) and (ΔS<0).
Reactions will be reversible if ΔH and ΔS have the same sign.
Gibbs Energy
Gibbs Free Energy
Mathematical combination of ΔH and ΔS. It is used to determine spontaneity of chemical reactions. Determines total useful energy available.
ΔG= ΔH-TΔS
Criteria
ΔG= -ve, the reaction is spontaneous and does useful work for the surroundings.
ΔG= +ve, reaction is non-spontaneous requires work from surroundings.
ΔG= 0, the reaction is at equilibrium.
Temperature Effect on ΔG
Low tempurature term is small, look at ΔH to determine if spontaneous.
High tempurature term is large, look at ΔS to determine if spontaneous.
If ΔH(-ve) and ΔS(+ve) are both spontaneous, then the reaction will be spontaneous no matter the temperature. (Opposite for non-spontaneous).
Introduction to Rates of Reactions
Chemical Kinetics- the study of the rates of chemical reactions
Reaction rate- the speed in which a chemical reaction occurs. It is determined by measuring
The rate at which a reactant is consumed.
The rate at which a product is produced.
Average and Instantaneous Reaction Rates
reaction rates are not constant, they vary with time
As reaction proceeds, the reactants decrease
As reaction proceeds, product increases
Average Rate of Reaction
Change in reactant or product over a given time interval. Can be determined by:
Using a data table
Determining the slope of a secant
Instantaneous Rate of Reaction
rate of reaction at a particular point in time
Determine by measuring the slope of a tangent
Reminder: Slope= rise/run
Measuring Reaction Rates
There are many properties that chemists can measure to find reaction rates. The method used depends on the nature of substance and the type or reaction. Measurements must be made without disturbing the progress of the reaction.
Change in Colour
some substances change colour as they produce new substances
Change in pH
if the H+ or OH- changes in the reactions, the pH will change.
Change in Electrical Conductivity
total number of ions changes from reactants to products. The ability of the system to conduct electricity is affected.
Changes in Volume or Pressure of Gas
if a gas is produced in a reaction. The change in volume/pressure over a given time period can be used to determine the rate.
Rate Laws and Order of Reaction
[ ] = concentration
Rate Law
rate of reaction [reactant]. As [reactant] increases, so does rate.
Rate law is the relationship between initial rate of reaction and initial concentration of reactant.
A balanced chemical equation is needed.
R=K[A]^x[B]^y
Rate Constant (k):
k is constant for any concentration of reactant at a given temp
The value of k varies with temp and is determined experimentally
Rate Law exponents (x and y)
do not vary with temp And are determined experimentally
Describe dépendance of reaction rate of initial concentration
Also called individual orders of reaction
Collision Theory
Factors that affect reaction rates:
Chemical Nature of Reactants
metals lower on the activity series are less reactive.
Reactions of monoatomic ions in aqueous solutions are very fast.
Reactions of molecular substances are often slow due to bonds needing to be broken and formed in the process.
Concentration
increasing initial concentration of reactants generally increases the reaction rates.
Temperature
increasing temp increases the reaction rate.
Rule of thumb: a 10C increase in temp doubles the reaction rate.
Catalyst
increases the reaction rate without being consumed.
Surface Area
increasing the surface area of solid reactants increases the reaction rate.
Collision Theory and Rates of Reaction
for a chemical reaction to occur, the reacting particles must collide with each other. But not every collision is effective. Not every collision leads to a reaction.
Conditions For An Effective Collision
Particles must collide with proper collision geometry.
Particles must collide with enough energy to break bonds in reacting particles.
Threshold Energy (Et)
minimum amount of Ek with which the reacting particles must collide to result in a reaction.
Activation Energy (Ea)
minimum amount of Ep that is required before the reactants can rearrange in structure and thus react.
An Effective Collision:
reacting particles collide with the proper collision geometry and sufficient energy so that bonds can break and new bonds can form.
An Ineffective Collision:
reacting particles rebound unchanged from the collision due to improper geometry and energy.
Collision Energy
depends on Ek of colliding particles
The higher the temp, the higher the Ek
Temp is the measure of average Ek
Maxwell-Boltzmann distribution curve- shows the relationship between the number of particles vs. Ek at a given temp. At a higher temp more particles have enough energy to overcome Et barrier.
Therefore reaction rate depends on:
Frequency of collision
Fraction of effective collisions
Rate= frequency x fraction
Concentration
increase of concentration→increases # of reacting particles
Increases frequency of collision and rate
Surface Area
Increase S.A→increases # of collisions
Increases frequency and rate
Temperature
Increase temp→increase spreed of particles
Increases frequency and fraction of effectiveness. Increases rate.
Nature of Reactants
effects fraction of effective collisions
Molecules with weak bonds have lower Ea, require less Ek to collide
Complicated molecules or ions require more Ek and less likely to collide in correct orientation.
Catalyst
provides alternative pathway with lower Ea→larger # of reactants have enough Ek to react.
# of effective collisions and rate increases.
Transistion State Theory
Used to explain what happens after molecules collide. And examines what happens as reactants change to products.
As reactants approach each other:
repulsive forces cause reactants to slow down
Ek converted to Ep
Et=Ea
Bond structure rearranges to form the product, only if enough Ep is stored.
As new molecules form, repulsive forces push molecules apart. Increase Ek.
Ea: difference between energy of reactants and the peak of the Ep curve.
H- difference between energy in reactants and products.
Activated Complex- unstable chemical species that contains partially broken and partially formed bonds. It represents the max Ep point. It’s formed only if reactants collide with enough energy and is formed during transition state.
 Knowt
Knowt
