Cape Biology Unit 1
Biomolecules
All major compounds that make up living organisms are based on the atom carbon, and are known as organic molecules
The body is comprised of many elements which combine to form molecules. These include macronutrients such as carbohydrates (such as starch and glucose, required for release of ATP), proteins (which are used for growth and repair of cells and also to form hormones) and fats (used for as an energy store).
Water
Majority of the human body is comprised of WATER (More than 70% of the cell’s mass).
Water is made up of two hydrogen atoms COVALENTLY bonded to one oxygen atom. This means that electrons shared between them.
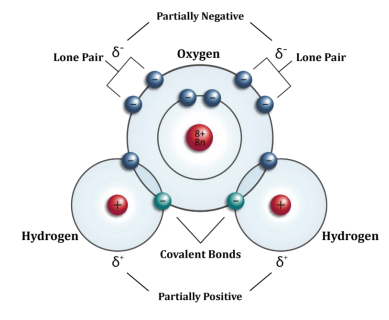
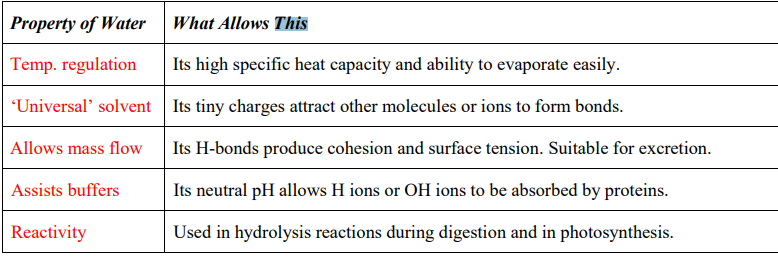
On the diagram, you will observe the symbol δ (delta), and a symbol for +ve or –ve charge. In this case, the OXYGEN has the negative charge and the HYDROGEN atoms have the positive charge. Water itself is electrically balanced or NEUTRAL. However, there is uneven distribution of these charges in the structure. This is called a DIPOLE. This allows weak electrical attraction between the water molecules, which results in COHESION and the ability to undego MASS FLOW. They also result in HYDROGEN BONDS, which are essential for many biological molecules.
Characteristics of Water:
Liquid at room temperature and pressure
Water molecules have no change due to the equal and particles charge (H20)
The oxygen molecules has a greater positivity than the hydrogen , attracting more hydrogen at the oxygen part.
The water molecules are strong and stable because they are covalently bonded. (intramolecular bonds)
Its polar for it has a partial negative part (O) and a partial positive part (H).
The weak intermolecular forces which are collected called hydrogen bonds sticky, waste, molecules.
Properties of Water:
Solvent Properties
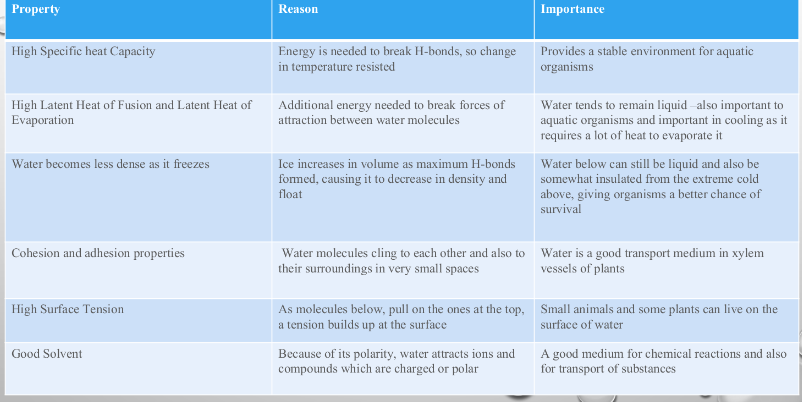
High Specific heat capacity
Hugh latent heat of evaporation/vaporization
High surface tension
Universal solvent
Roles of Water
Solvent Properties
This is because water consist of one oxygen and two hydrogen atoms forming strong intra-molecular (within the molecule) bonds. This makes the water molecule a very stable compound. The water molecule therefore has no overall charge, but because the O has many more protons in the nucleus, It attracts more electrons than the H atom which makes it a polar molecule, this means it has a partial positive side which is the hydrogen which attracts negatively charged ions and partial negative side which attracts partially positive ions causes it surround and pull apart bonds by attraction the ions bonded together which dissociates the bonds of the solute making water to be an excellent solvent. Water attracts ions and compounds which are charged or polar
High Heat Capacity
Water has a high heat capacity for a large increase in heat energy results in a relatively rise in temperature. Thus temperature change in water minimized by the heat capacity. This is because much of the energy is used in breaking the hydrogen bonds (overcoming the ‘stickiness’) which restrict the movement of the molecules. Thus water provides a very constant environment for many cells and organisms.
High Heat of Vaporization
A relatively large amount of energy is needed to evaporate or boil into a gas. This is due to the hydrogen bonding as a resulting in water having a high boiling point.
High Heat of Fusion
Because of water’s high heat capacity water requires relatively large amounts of heat energy for it to melt it in its solid form. Conversely, liquids must lose a relatively large amount of heat energy to freeze. Contents of cells and their environments are therefore less likely to freeze. Ice crystals are particularly damaging if develop inside cells.
Density and Freezing Properties
Study Notes
Polarity is an uneven charge distribution within a molecule. In water.
In water one part, or pole of the molecules is slightly positive while the other side is slightly negative. This is known as a dipole. It occurs because the oxygen atom has greater electron attracting power than hydrogen atoms. As a result the oxygen atoms tend to attract the single electrons of the hydrogen atoms. Electrons are negatively charge relative to the hydrogen atom,
They are not permanent bonds
The presence of hydrogen bonds explain the high cohesive strength of water (they stick together). It is this cohesive strength that permits narrow columns of water in xylem of plants to reach the top of trees. Transpiration causes the entire column of water to move upwards in responses to the pull of water molecules.
Due to the weak attraction water molecules have for each other the opposite charges coming together and behave as if they are ‘sticky’.
These attractions are not as strong as normal ionic bonds called hydrogen bonds.
Water has a high surface tension. This means that the surface between liquid and water and air is relatively difficult to puncture because a lot of energy is needed to force the hydrogen-bonded water molecules away from one another.
Surface tension allows a container to be filled slightly above its rim without overflowing.
When water freezes, it forms a crystalline structure. In ice, each water molecule is hydrogen boned to 4 other molecules in a 3-dimensional lattice.
Water molecules in ice crystals are fixed in an hexagonal arrangement.
Water at a temperature between 4°C and 0°C is less dense than water .
The maximum density for water is achieved at 4°C.
To melt ice requires the input of a large amount of heat energy. The value is high because of the high number of hydrogen bonds that must be broken to change ice into liquid water. The opposite happens - giving to the environment - when changing from water to ice.
Water is a good solvent for polar or charged molecules.
Nucleic Acids
The tern ‘nucleic acid’ comes from the fact that they are found mainly in the nucleus.
DNA stands for DEOXYRIBONUCLEIC ACID and RNA stands for RIBONUCLEIC ACID. This is because DNA lacks an OXYGEN that RNA has. They are mainly found in the NUCLEUS of the cells and their tasks are to produce a genetic code to express certain traits, such as eye color, blood type and whether or not a disease is present, such as hemophilia.
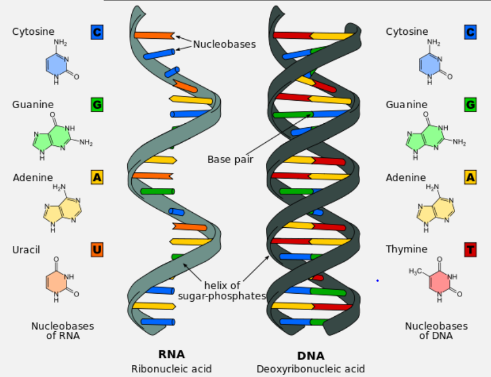
DNA has the shape of a DOUBLE HELIX. Each chain of this helix is made of NUCLEOTIDES, which each have organic BASES that are connected by HYDROGEN bonds.
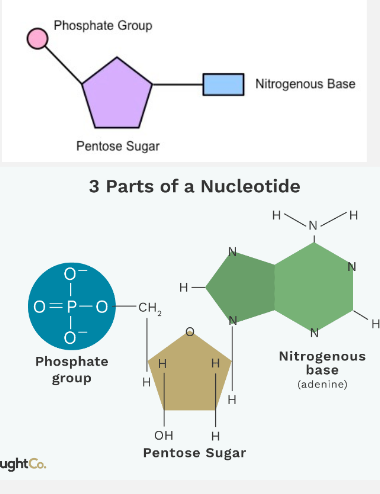
A single nucleotide is made up of:
A phosphate group covalently bonded to the 5’ Carbon on the sugar
A pentose sugar, deoxyribose or ribose
Deoxyribose has one less Oxygen than ribose, so the 2’ carbon has an H attached on deoxyribose, while the 2’ carbon on the ribose sugar has OH
Both are 5 sided rings with 5 carbons. Oxygen occupies one position, so 4 carbons are in the ring and the 5th is above the ring
A nitrogenous base which is covalently bonded to the I‘ Carbon. There are 4 different nitrogenous bases for each nucleic acid (A, C, T, G)
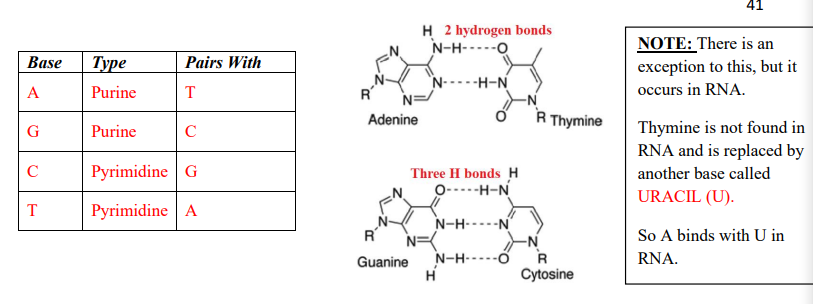
A and G have two rings and are called PURINES
C and T have one ring and are called PYRIMIDINESS
These form COMPLEMENTARY BASE PAIRS and are linked by hydrogen bonds. Only a purine can bond with a pyrimidine
Thus:
A can only pair with T and U (which is on RNA) (Apple in a Tree)
C can only pair with G (Car in the Garage)
DNA and RNA each have 4 bases. Both have A C G and DNA has T as its 4th base, while RNA has U instead.
The bases are attached to each other by Hydrogen Bonds, which are individually very weak, easily formed and broken, but collectively strong.
(This is a very important feature of how DNA and RNA carry out their function.
Note that there are 3 H-bonds between C and G and 2 H-bonds between A and T
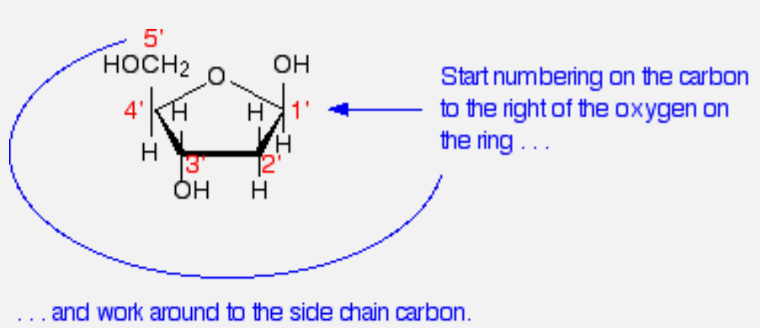
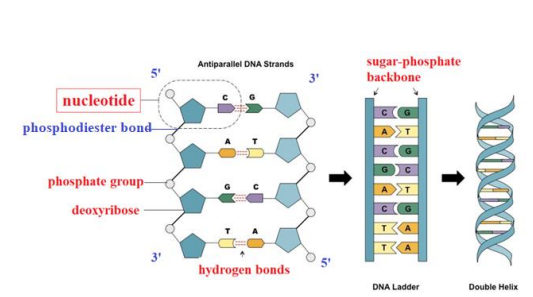 In order for the bases to become attached to each other, the nucleotides in the second strand must be rotated, so that the second strand goes in the opposite direction. From the diagram, you will notice numbers marked 3‟ and 5‟. This relates to how the PHOSPHATES are connected. 5‟ means it is connected to the 5th carbon (just off the deoxyribose ring). 3‟ means it is connected to the 3rd carbon. When the phosphate links with the sugar, it forms a PHOSPHODIESTER bond. This is a CONDENSATION reaction. As said before both chains actually run in opposite directions (notice the inverted sugars). They are thus said to be ANTIPARALLEL.
In order for the bases to become attached to each other, the nucleotides in the second strand must be rotated, so that the second strand goes in the opposite direction. From the diagram, you will notice numbers marked 3‟ and 5‟. This relates to how the PHOSPHATES are connected. 5‟ means it is connected to the 5th carbon (just off the deoxyribose ring). 3‟ means it is connected to the 3rd carbon. When the phosphate links with the sugar, it forms a PHOSPHODIESTER bond. This is a CONDENSATION reaction. As said before both chains actually run in opposite directions (notice the inverted sugars). They are thus said to be ANTIPARALLEL.
DNA and RNA are both nucleic acids.
DNA carries the genetic code, which are a set of instructions to make proteins
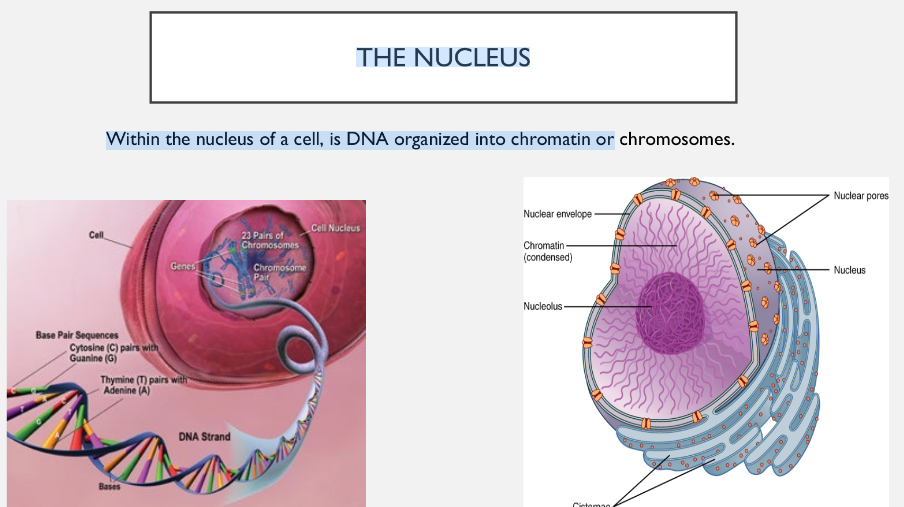
DNA is always found in the nucleus of eukaryotic cells, and in the nucleoid of prokaryotic cells.
Prokaryotes also have tiny rings of DNA called plasmids
Sections of DNA which carry the code for making a particular polypeptide are called genes.
Nuclear DNA is condensed into either chromatin or chromosomes
DNA is also found in mitochondria and chloroplasts
RNA is responsible for copying the code and using it to assemble polypeptides
RNA is found in the cytoplasm and there are three different types.
DNA AND RNA STRCTURE
DNA and RNA are both poly-nucleotides, made up of thousands of nucleotides bonded together, by condensation, forming phospho-diester bonds, which are covalent bonds, between the phosphate group of one nucleotide and the 3’ Carbon of another.
DNA is made up of two strands, the “backbone” being formed by the sugar/phosphate parts, while the bases form the rungs.
RNA is always single-stranded.
 TYPES OF RNA
TYPES OF RNA
mRNA (messenger RNA)- this RNA that carries information from DNA to the ribosomes sites of protein synthesis in the cell.
tRNA (transfer RNA)- this is a small RNA chain of about 74-93 nucleotides that carries amino acids tot he location of the protein synthesis.

rRNA (ribosomal RNA)- this is a component of the Ribosomes, the protein synthesis synthetic factories in the cell.

DNA and RNA Structure Compared
DNA | RNA | |
|---|---|---|
Pentose sugar | Deoxyribose | Ribose |
Base Composition | Adenine (A)Guanine (G)Cytosine (C)Thymine (T) | Adenine (A)Guanine (G)Cytosine (C)Uracil (U) |
Number of strands | Double stranded (Forms a double helix | Single stranded |
Location | Nucleus | Nucleus and cytoplasm |
Role | Storage of genetic code | Copying and transfer of code for DNA to ribosomes to syntehsize proteins |
DNA REPLICATION, TRANSCRIPTION AND TRANSLATION
In order for a cell to divide into two equal parts, the entire DNA must be doubled – this is DNA Replication.
It can be seen that after Replication the chromosome number does not change, but each chromosome has twice the amount of DNA. Upon cell division each daughter cell can now have identical chromosomes to the parent cell and to each other.
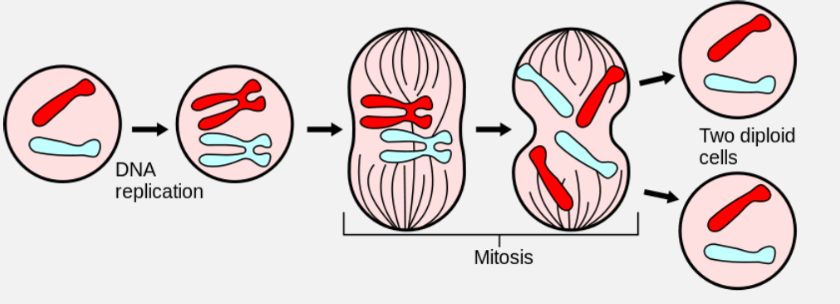
SEMI-CONSERVATIVE REPLICATION
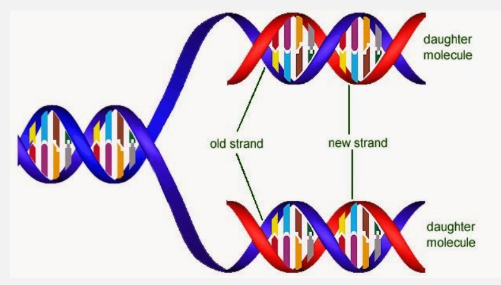
DNA replication is semi-conservative, which means that the two new molecules of DNA have kept one strand from the parent DNA and added a new one. At the end of replication, the original DNA no longer exists, as it has been incorporated into the new DNA.
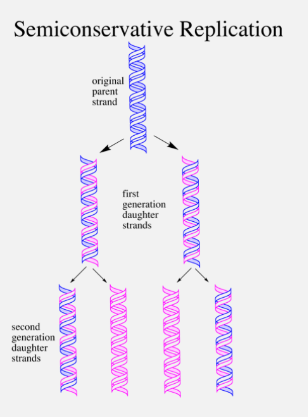
Replication Fork
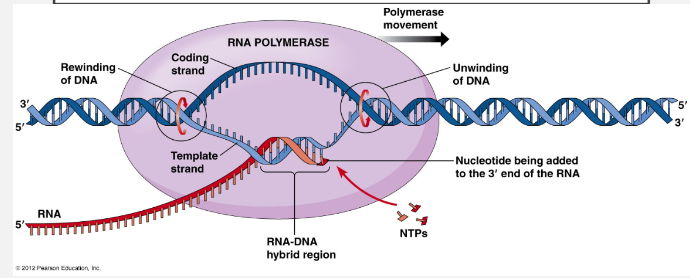
The DNA strand unzips one section at a time, forming a Replication Fork.
In order to add the complementary nucleotides, each parent strand is read in the 3’ 5’ direction and nucleotides added in the 5’ 3’ direction (anti-parallel) The nucleotides that are being added in the direction of the unzipping can be added continuously and is called the leading strand.
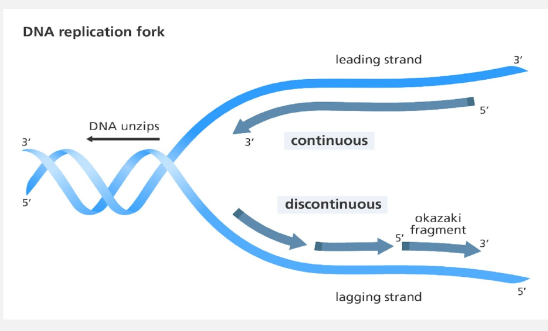
The nucleotides that are being added in the other direction have to wait until the unzipping takes place and are added in pieces, called Okazaki Fragments. This strand is called the lagging strand.
STAGES IN REPLICATION
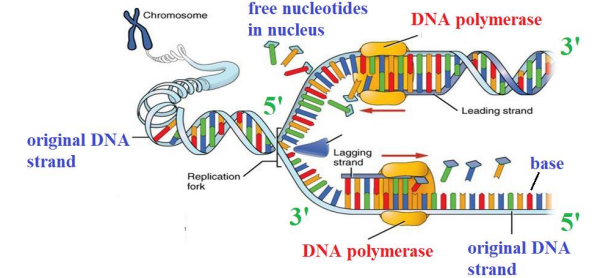
In enzyme known as DNA HELICASE “Unzips‟ the DNA into two strands by breaking the HYDROGEN bonds between the bases. There is now a „leading‟ and „lagging‟ strand. Both exposed stands act as templates and attract complementary nucleotides in A-T, C-G pattern.
Another enzyme known as DNA POLYMERASE slides along the strands in 3’ 5’ direction and nucleotides are added in the 5’ 3’ direction, continuously on the leading strand and in fragments on the lagging strand.
DNA Ligase joins up the backbones of the Okazaki Fragments and HYDROGEN bonds form, to the original strand, and there are now two DNA molecules. The new DNA formed will wind up into two double helices, each having an old and a new strand (semi-conservative)
When replication is complete, we end up two identical DNA molecules.

Each molecule contains 1 original strand and 1 new strand. The original DNA will not exist anymore.
HOW WAS SEMICONSERVATIVE REPLICATION PROVEN?

The concept was proven by two scientists named Meselson and Stahl. They submerged E. coli bacteria in ammonium chloride with the nitrogen isotope being ‘dense’ (N-15). This meant that this isotope was all the bacteria should’ve had N-15 in its DNA. The cells were then transferred to a medium containing the isotope N-14, which is ‘less dense’. The bacteria were harvested and the DNA collected and dissolved in caesium chloride (CsCl). This was then put in a centrifuge and a concentration gradient was established. Observe the diagram below. After 1 generation, it showed a band of DNA of intermediate density.
This means that it contained a strand of both the N-14 and N-15 DNA. This proved that DNA was built by new nucleotides to form complementary strands on the new templates.
WHAT ARE RIBOSOMES?
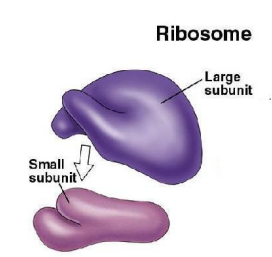
Ribosomes synthesize proteins. They are found in both eukaryotes and prokaryotes. They are found held onto the ROUGH ER. They contain ribosomal RNA or rRNA and some protein. Think of ribosomes as having two parts, a large and small sub-unit. The large sub-unit helps hold substances like mRNA in place while the smaller sub-unit is more flexible. rRNA can catalyze the formation of PEPTIDE bonds between amino acids when synthesizing proteins.
HOW GENETIC CODE WORKS?
A Gene is a unit of hereditary material made up of a section of DNA that codes for a polypeptide. A GENE is the length of DNA that codes for a single polypeptide. A tiny alteration in the sequence of the DNA can cause a large change in the protein synthesized. If this occurs randomly (usually due to a ‘copying error’), it is called a MUTATION.A gene is therefore responsible for making proteins, many of which are enzymes and so they regulate the growth, development and daily functioning of cells.
All DNA nucleotides have the same sugar and phosphate but there are 4 different bases.
The genetic code must therefore lie in the bases. There are 4 bases and 20 amino acids, so
A single base could only code for 4 amino acids, which is not enough 4x1 =4
Combinations of 2 bases could code for 16 amino acids, which is almost but still not enough 4x4=16
Combinations of 3 bases could code for 64 amino acids, which is more than enough. 4x4x4 = 64
The genetic code therefore has the following features:
It is a triplet code – 3 bases code for one amino acid
It is universal – the code means the same in all organisms
It is degenerate – it is excessive as there are 64 triplet codes and only 20 amino acids
The code is non-overlapping and non-punctuated
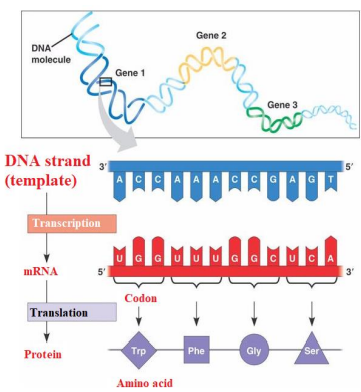
TRANSCRIPTION – The DNA code is copied onto a molecule called MESSENGER RNA (mRNA). This is done three bases at a time, called a base TRIPLET or a CODON. Remember that RNA does not have thymine (T) so adenine (A) on the coding strands is transcripted as URACIL (U) on the mRNA.
The mRNA will leave the nucleus and go into the cytoplasm, taking the genetic code with it. The triplet code has been converted to complementary bases, which are called Codons.
Ribosomes with rRNA will attach to the mRNA, so translation can occur.
Cape Biology Unit 1
Biomolecules
All major compounds that make up living organisms are based on the atom carbon, and are known as organic molecules
The body is comprised of many elements which combine to form molecules. These include macronutrients such as carbohydrates (such as starch and glucose, required for release of ATP), proteins (which are used for growth and repair of cells and also to form hormones) and fats (used for as an energy store).
Water
Majority of the human body is comprised of WATER (More than 70% of the cell’s mass).
Water is made up of two hydrogen atoms COVALENTLY bonded to one oxygen atom. This means that electrons shared between them.

On the diagram, you will observe the symbol δ (delta), and a symbol for +ve or –ve charge. In this case, the OXYGEN has the negative charge and the HYDROGEN atoms have the positive charge. Water itself is electrically balanced or NEUTRAL. However, there is uneven distribution of these charges in the structure. This is called a DIPOLE. This allows weak electrical attraction between the water molecules, which results in COHESION and the ability to undego MASS FLOW. They also result in HYDROGEN BONDS, which are essential for many biological molecules.
Characteristics of Water:
Liquid at room temperature and pressure
Water molecules have no change due to the equal and particles charge (H20)
The oxygen molecules has a greater positivity than the hydrogen , attracting more hydrogen at the oxygen part.
The water molecules are strong and stable because they are covalently bonded. (intramolecular bonds)
Its polar for it has a partial negative part (O) and a partial positive part (H).
The weak intermolecular forces which are collected called hydrogen bonds sticky, waste, molecules.
Properties of Water:
Solvent Properties
High Specific heat capacity
Hugh latent heat of evaporation/vaporization
High surface tension
Universal solvent

Roles of Water
Solvent Properties
This is because water consist of one oxygen and two hydrogen atoms forming strong intra-molecular (within the molecule) bonds. This makes the water molecule a very stable compound. The water molecule therefore has no overall charge, but because the O has many more protons in the nucleus, It attracts more electrons than the H atom which makes it a polar molecule, this means it has a partial positive side which is the hydrogen which attracts negatively charged ions and partial negative side which attracts partially positive ions causes it surround and pull apart bonds by attraction the ions bonded together which dissociates the bonds of the solute making water to be an excellent solvent. Water attracts ions and compounds which are charged or polar
High Heat Capacity
Water has a high heat capacity for a large increase in heat energy results in a relatively rise in temperature. Thus temperature change in water minimized by the heat capacity. This is because much of the energy is used in breaking the hydrogen bonds (overcoming the ‘stickiness’) which restrict the movement of the molecules. Thus water provides a very constant environment for many cells and organisms.
High Heat of Vaporization
A relatively large amount of energy is needed to evaporate or boil into a gas. This is due to the hydrogen bonding as a resulting in water having a high boiling point.
High Heat of Fusion
Because of water’s high heat capacity water requires relatively large amounts of heat energy for it to melt it in its solid form. Conversely, liquids must lose a relatively large amount of heat energy to freeze. Contents of cells and their environments are therefore less likely to freeze. Ice crystals are particularly damaging if develop inside cells.
Density and Freezing Properties
Study Notes
Polarity is an uneven charge distribution within a molecule. In water.
In water one part, or pole of the molecules is slightly positive while the other side is slightly negative. This is known as a dipole. It occurs because the oxygen atom has greater electron attracting power than hydrogen atoms. As a result the oxygen atoms tend to attract the single electrons of the hydrogen atoms. Electrons are negatively charge relative to the hydrogen atom,
They are not permanent bonds
The presence of hydrogen bonds explain the high cohesive strength of water (they stick together). It is this cohesive strength that permits narrow columns of water in xylem of plants to reach the top of trees. Transpiration causes the entire column of water to move upwards in responses to the pull of water molecules.
Due to the weak attraction water molecules have for each other the opposite charges coming together and behave as if they are ‘sticky’.
These attractions are not as strong as normal ionic bonds called hydrogen bonds.
Water has a high surface tension. This means that the surface between liquid and water and air is relatively difficult to puncture because a lot of energy is needed to force the hydrogen-bonded water molecules away from one another.
Surface tension allows a container to be filled slightly above its rim without overflowing.
When water freezes, it forms a crystalline structure. In ice, each water molecule is hydrogen boned to 4 other molecules in a 3-dimensional lattice.
Water molecules in ice crystals are fixed in an hexagonal arrangement.
Water at a temperature between 4°C and 0°C is less dense than water .
The maximum density for water is achieved at 4°C.
To melt ice requires the input of a large amount of heat energy. The value is high because of the high number of hydrogen bonds that must be broken to change ice into liquid water. The opposite happens - giving to the environment - when changing from water to ice.
Water is a good solvent for polar or charged molecules.
Nucleic Acids
The tern ‘nucleic acid’ comes from the fact that they are found mainly in the nucleus.
DNA stands for DEOXYRIBONUCLEIC ACID and RNA stands for RIBONUCLEIC ACID. This is because DNA lacks an OXYGEN that RNA has. They are mainly found in the NUCLEUS of the cells and their tasks are to produce a genetic code to express certain traits, such as eye color, blood type and whether or not a disease is present, such as hemophilia.
DNA has the shape of a DOUBLE HELIX. Each chain of this helix is made of NUCLEOTIDES, which each have organic BASES that are connected by HYDROGEN bonds.
A single nucleotide is made up of:
A phosphate group covalently bonded to the 5’ Carbon on the sugar
A pentose sugar, deoxyribose or ribose
Deoxyribose has one less Oxygen than ribose, so the 2’ carbon has an H attached on deoxyribose, while the 2’ carbon on the ribose sugar has OH
Both are 5 sided rings with 5 carbons. Oxygen occupies one position, so 4 carbons are in the ring and the 5th is above the ring
A nitrogenous base which is covalently bonded to the I‘ Carbon. There are 4 different nitrogenous bases for each nucleic acid (A, C, T, G)

A and G have two rings and are called PURINES
C and T have one ring and are called PYRIMIDINESS
These form COMPLEMENTARY BASE PAIRS and are linked by hydrogen bonds. Only a purine can bond with a pyrimidine
Thus:
A can only pair with T and U (which is on RNA) (Apple in a Tree)
C can only pair with G (Car in the Garage)
DNA and RNA each have 4 bases. Both have A C G and DNA has T as its 4th base, while RNA has U instead.
The bases are attached to each other by Hydrogen Bonds, which are individually very weak, easily formed and broken, but collectively strong.
(This is a very important feature of how DNA and RNA carry out their function.
Note that there are 3 H-bonds between C and G and 2 H-bonds between A and T

 In order for the bases to become attached to each other, the nucleotides in the second strand must be rotated, so that the second strand goes in the opposite direction. From the diagram, you will notice numbers marked 3‟ and 5‟. This relates to how the PHOSPHATES are connected. 5‟ means it is connected to the 5th carbon (just off the deoxyribose ring). 3‟ means it is connected to the 3rd carbon. When the phosphate links with the sugar, it forms a PHOSPHODIESTER bond. This is a CONDENSATION reaction. As said before both chains actually run in opposite directions (notice the inverted sugars). They are thus said to be ANTIPARALLEL.
In order for the bases to become attached to each other, the nucleotides in the second strand must be rotated, so that the second strand goes in the opposite direction. From the diagram, you will notice numbers marked 3‟ and 5‟. This relates to how the PHOSPHATES are connected. 5‟ means it is connected to the 5th carbon (just off the deoxyribose ring). 3‟ means it is connected to the 3rd carbon. When the phosphate links with the sugar, it forms a PHOSPHODIESTER bond. This is a CONDENSATION reaction. As said before both chains actually run in opposite directions (notice the inverted sugars). They are thus said to be ANTIPARALLEL.
DNA and RNA are both nucleic acids.
DNA carries the genetic code, which are a set of instructions to make proteins
DNA is always found in the nucleus of eukaryotic cells, and in the nucleoid of prokaryotic cells.
Prokaryotes also have tiny rings of DNA called plasmids
Sections of DNA which carry the code for making a particular polypeptide are called genes.
Nuclear DNA is condensed into either chromatin or chromosomes
DNA is also found in mitochondria and chloroplasts
RNA is responsible for copying the code and using it to assemble polypeptides
RNA is found in the cytoplasm and there are three different types.


DNA AND RNA STRCTURE
DNA and RNA are both poly-nucleotides, made up of thousands of nucleotides bonded together, by condensation, forming phospho-diester bonds, which are covalent bonds, between the phosphate group of one nucleotide and the 3’ Carbon of another.
DNA is made up of two strands, the “backbone” being formed by the sugar/phosphate parts, while the bases form the rungs.
RNA is always single-stranded.
 TYPES OF RNA
TYPES OF RNA
mRNA (messenger RNA)- this RNA that carries information from DNA to the ribosomes sites of protein synthesis in the cell.

tRNA (transfer RNA)- this is a small RNA chain of about 74-93 nucleotides that carries amino acids tot he location of the protein synthesis.

rRNA (ribosomal RNA)- this is a component of the Ribosomes, the protein synthesis synthetic factories in the cell.

DNA and RNA Structure Compared
DNA | RNA | |
|---|---|---|
Pentose sugar | Deoxyribose | Ribose |
Base Composition | Adenine (A)Guanine (G)Cytosine (C)Thymine (T) | Adenine (A)Guanine (G)Cytosine (C)Uracil (U) |
Number of strands | Double stranded (Forms a double helix | Single stranded |
Location | Nucleus | Nucleus and cytoplasm |
Role | Storage of genetic code | Copying and transfer of code for DNA to ribosomes to syntehsize proteins |
DNA REPLICATION, TRANSCRIPTION AND TRANSLATION
In order for a cell to divide into two equal parts, the entire DNA must be doubled – this is DNA Replication.
It can be seen that after Replication the chromosome number does not change, but each chromosome has twice the amount of DNA. Upon cell division each daughter cell can now have identical chromosomes to the parent cell and to each other.

SEMI-CONSERVATIVE REPLICATION

DNA replication is semi-conservative, which means that the two new molecules of DNA have kept one strand from the parent DNA and added a new one. At the end of replication, the original DNA no longer exists, as it has been incorporated into the new DNA.

Replication Fork
The DNA strand unzips one section at a time, forming a Replication Fork.
In order to add the complementary nucleotides, each parent strand is read in the 3’ 5’ direction and nucleotides added in the 5’ 3’ direction (anti-parallel) The nucleotides that are being added in the direction of the unzipping can be added continuously and is called the leading strand.

The nucleotides that are being added in the other direction have to wait until the unzipping takes place and are added in pieces, called Okazaki Fragments. This strand is called the lagging strand.
STAGES IN REPLICATION

In enzyme known as DNA HELICASE “Unzips‟ the DNA into two strands by breaking the HYDROGEN bonds between the bases. There is now a „leading‟ and „lagging‟ strand. Both exposed stands act as templates and attract complementary nucleotides in A-T, C-G pattern.
Another enzyme known as DNA POLYMERASE slides along the strands in 3’ 5’ direction and nucleotides are added in the 5’ 3’ direction, continuously on the leading strand and in fragments on the lagging strand.
DNA Ligase joins up the backbones of the Okazaki Fragments and HYDROGEN bonds form, to the original strand, and there are now two DNA molecules. The new DNA formed will wind up into two double helices, each having an old and a new strand (semi-conservative)
When replication is complete, we end up two identical DNA molecules.

Each molecule contains 1 original strand and 1 new strand. The original DNA will not exist anymore.
HOW WAS SEMICONSERVATIVE REPLICATION PROVEN?

The concept was proven by two scientists named Meselson and Stahl. They submerged E. coli bacteria in ammonium chloride with the nitrogen isotope being ‘dense’ (N-15). This meant that this isotope was all the bacteria should’ve had N-15 in its DNA. The cells were then transferred to a medium containing the isotope N-14, which is ‘less dense’. The bacteria were harvested and the DNA collected and dissolved in caesium chloride (CsCl). This was then put in a centrifuge and a concentration gradient was established. Observe the diagram below. After 1 generation, it showed a band of DNA of intermediate density.
This means that it contained a strand of both the N-14 and N-15 DNA. This proved that DNA was built by new nucleotides to form complementary strands on the new templates.
WHAT ARE RIBOSOMES?

Ribosomes synthesize proteins. They are found in both eukaryotes and prokaryotes. They are found held onto the ROUGH ER. They contain ribosomal RNA or rRNA and some protein. Think of ribosomes as having two parts, a large and small sub-unit. The large sub-unit helps hold substances like mRNA in place while the smaller sub-unit is more flexible. rRNA can catalyze the formation of PEPTIDE bonds between amino acids when synthesizing proteins.
HOW GENETIC CODE WORKS?
A Gene is a unit of hereditary material made up of a section of DNA that codes for a polypeptide. A GENE is the length of DNA that codes for a single polypeptide. A tiny alteration in the sequence of the DNA can cause a large change in the protein synthesized. If this occurs randomly (usually due to a ‘copying error’), it is called a MUTATION.A gene is therefore responsible for making proteins, many of which are enzymes and so they regulate the growth, development and daily functioning of cells.
All DNA nucleotides have the same sugar and phosphate but there are 4 different bases.
The genetic code must therefore lie in the bases. There are 4 bases and 20 amino acids, so
A single base could only code for 4 amino acids, which is not enough 4x1 =4
Combinations of 2 bases could code for 16 amino acids, which is almost but still not enough 4x4=16
Combinations of 3 bases could code for 64 amino acids, which is more than enough. 4x4x4 = 64
The genetic code therefore has the following features:
It is a triplet code – 3 bases code for one amino acid
It is universal – the code means the same in all organisms
It is degenerate – it is excessive as there are 64 triplet codes and only 20 amino acids
The code is non-overlapping and non-punctuated

TRANSCRIPTION – The DNA code is copied onto a molecule called MESSENGER RNA (mRNA). This is done three bases at a time, called a base TRIPLET or a CODON. Remember that RNA does not have thymine (T) so adenine (A) on the coding strands is transcripted as URACIL (U) on the mRNA.
The mRNA will leave the nucleus and go into the cytoplasm, taking the genetic code with it. The triplet code has been converted to complementary bases, which are called Codons.
Ribosomes with rRNA will attach to the mRNA, so translation can occur.

 Knowt
Knowt