
CHAPTER 17: ADDITIONAL ASPECTS OF AQUEOUS EQUILIBRIA
The Common-Ion Effect
Is the phenomenon in which the addition of a common ion to a solution causes the solubility of an ionic compound to decrease.
This is because the common ion competes with the ionic compound for the same ions in solution, thus reducing the amount of the ionic compound that can dissolve.
The common-ion effect is an important concept in chemistry, as it can be used to explain the solubility of ionic compounds in different solutions.
Buffers
Buffered solutions
(or merely buffers)
Solutions that contain high concentrations (10-3 M or more) of a weak conjugate acid–base pair and that resist drastic changes in pH when small amounts of strong acid or strong base are added to them.
Composition and Action of Buffers
A buffer is a solution that resists changes in pH when acids or bases are added. Buffers are composed of a weak acid and its conjugate base, or a weak base and its conjugate acid. Buffers work by releasing or absorbing hydrogen ions to maintain a constant pH. Buffers are important in many biological and chemical processes, as they help to maintain a stable environment.
Calculating the pH of a Buffer
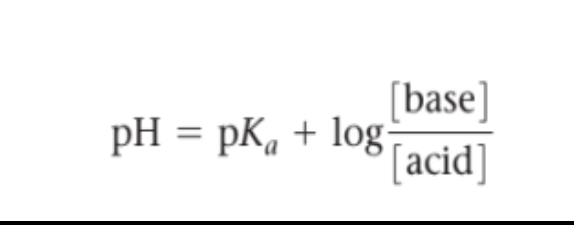
Henderson–Hasselbalch equation
Is a mathematical equation used to calculate the pH of a solution given the concentrations of the acid and its conjugate base. It is used to determine the equilibrium pH of a solution containing an acid and its conjugate base.
The equation is pH = pKa + log([base]/[acid]).
Buffer Capacity and pH Range
Buffer capacity is the amount of acid or base the buffer can neutralize before the pH begins to change to an appreciable degree. The buffer capacity depends on the amount of acid and base used to prepare the buffer.
The pH range of any buffer is the pH range over which the buffer acts effectively. Buffers most effectively resist a change in pH in either direction when the concentrations of weak acid and conjugate base are about the same.
Acid–Base Titrations
Titrations are procedures in which one reactant is slowly added into a solution of another reactant, while equilibrium concentrations along the way are monitored.
There are two main reasons to do titrations:
To determine the concentration of one of the reactants
To determine the equilibrium constant for the reaction
Acid–base indicators can be used to signal the equivalence point of a titration (the point at which stoichiometrically equivalent quantities of acid and base have been brought together).
pH titration curve
Graph of the pH as a function of the volume of titrant added. The shape of the titration curve makes it possible to determine the equivalence point.
Strong Acid–Strong Base Titrations
Are a type of titration used to determine the concentration of an acid or base in a solution. This type of titration involves the use of a strong acid and a strong base, and the endpoint is determined by the indicator used.
Weak Acid–Strong Base Titrations
Are a type of titration used to determine the concentration of an acid or base in a solution. In this type of titration, a strong base is added to a weak acid until the acid is completely neutralized. The amount of base added is then used to calculate the concentration of the acid. This type of titration Is useful for determining the concentration of weak acids, such as acetic acid, in a solution.
The titration curve for a weak acid–strong base titration differs from the curve for a strong acid–strong base titration in three noteworthy ways:
The solution of the weak acid has a higher initial pH than a solution of a strong acid of the same concentration.
The pH change in the rapid-rise portion of the curve near the equivalence point is smaller for the weak acid than for the strong acid.
The pH at the equivalence point is above 7.00 for the weak acid titration.
Titrating with an Acid–Base Indicator
An indicator is a compound that changes color in solution over a specific pH range. Optimally, an indicator should change color at the equivalence point in a titration.
The point in a titration where the indicator changes color is called the end point to distinguish it from the equivalence point that it closely approximates.
Solubility Equilibria
Solubility-product constant
The equilibrium constant indicates how soluble the solid is in water.
The coefficient for each ion in the equilibrium equation also equals its subscript in the compound’s chemical formula.
Molar solubility
Is the number of moles of solute that dissolve in forming 1 L of saturated solution of the solute (mol/L).
Factors That Affect Solubility
Solubility is affected by temperature and by the presence of other solutes. The presence of an acid, for example, can have a major influence on the solubility of a substance.
The Common-Ion Effect
Is a phenomenon in chemistry that occurs when a common ion is added to a solution.
This can cause the solubility of a compound to decrease, or the pH of the solution to change.
The Common-Ion Effect is important to consider when making solutions, as it can affect the concentration of the solution and the reaction rate of the compounds in the solution.
Solubility and pH
The solubility of almost any ionic compound is affected if the solution is made sufficiently acidic or basic. The effects are noticeable, however, only when one (or both) ions in the compound is at least moderately acidic or basic.
In general, the solubility of a compound containing a basic anion (that is, the anion of a weak acid) increases as the solution becomes more acidic.
Formation of Complex Ions
A characteristic property of metal ions is their ability to act as Lewis acids toward water molecules, which act as Lewis bases.Lewis bases other than water can also interact with metal ions, particularly transition-metal ions. Such interactions can dramatically affect the solubility of a metal salt.
Complex ion
A complex ion is a molecule or ion composed of two or more atoms held together by chemical bonds.Complex ions are formed when a metal atom or ion binds to one or more molecules or ions.
Formation constant
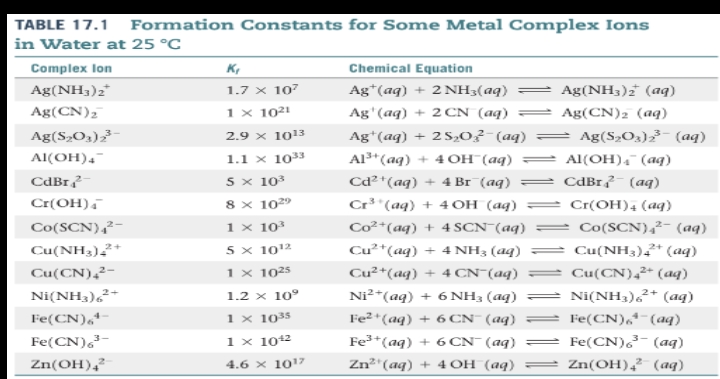
Amphoterism
Some metal oxides and hydroxides that are relatively insoluble in water dissolve in strongly acidic and strongly basic solutions. These substances, called amphoteric oxides and amphoteric hydroxides,are soluble in strong acids and bases because they themselves are capable of behaving as either an acid or base.
Precipitation and Separation of Ions
Equilibrium can be achieved starting with the substances on either side of a chemical equation.
Selective Precipitation of Ions
Is a process used to separate ions from a solution based on their solubility.
This process involves adding a reagent to the solution that will cause certain ions to precipitate out of the solution, while leaving other ions in the solution.
This process is often used in industrial processes to separate ions from a solution for further processing.
Qualitative Analysis for Metallic Elements
Qualitative analysis determines only the presence or absence of a particular metal ion relative to some threshold, whereas quantitative analysis determines how much of a given substance is present.
Typically, such analyses proceed in three stages:
(1) The ions are separated into broad groups on the basis of solubility properties.
(2) The ions in each group are separated by selectively dissolving members in the group.
(3) The ions are identified by means of specific tests.
CHAPTER 17: ADDITIONAL ASPECTS OF AQUEOUS EQUILIBRIA
The Common-Ion Effect
Is the phenomenon in which the addition of a common ion to a solution causes the solubility of an ionic compound to decrease.
This is because the common ion competes with the ionic compound for the same ions in solution, thus reducing the amount of the ionic compound that can dissolve.
The common-ion effect is an important concept in chemistry, as it can be used to explain the solubility of ionic compounds in different solutions.
Buffers
Buffered solutions
(or merely buffers)
Solutions that contain high concentrations (10-3 M or more) of a weak conjugate acid–base pair and that resist drastic changes in pH when small amounts of strong acid or strong base are added to them.
Composition and Action of Buffers
A buffer is a solution that resists changes in pH when acids or bases are added. Buffers are composed of a weak acid and its conjugate base, or a weak base and its conjugate acid. Buffers work by releasing or absorbing hydrogen ions to maintain a constant pH. Buffers are important in many biological and chemical processes, as they help to maintain a stable environment.
Calculating the pH of a Buffer
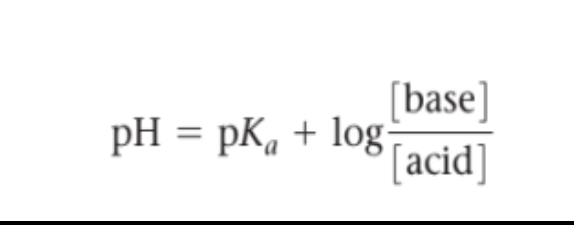
Henderson–Hasselbalch equation
Is a mathematical equation used to calculate the pH of a solution given the concentrations of the acid and its conjugate base. It is used to determine the equilibrium pH of a solution containing an acid and its conjugate base.
The equation is pH = pKa + log([base]/[acid]).
Buffer Capacity and pH Range
Buffer capacity is the amount of acid or base the buffer can neutralize before the pH begins to change to an appreciable degree. The buffer capacity depends on the amount of acid and base used to prepare the buffer.
The pH range of any buffer is the pH range over which the buffer acts effectively. Buffers most effectively resist a change in pH in either direction when the concentrations of weak acid and conjugate base are about the same.
Acid–Base Titrations
Titrations are procedures in which one reactant is slowly added into a solution of another reactant, while equilibrium concentrations along the way are monitored.
There are two main reasons to do titrations:
To determine the concentration of one of the reactants
To determine the equilibrium constant for the reaction
Acid–base indicators can be used to signal the equivalence point of a titration (the point at which stoichiometrically equivalent quantities of acid and base have been brought together).
pH titration curve
Graph of the pH as a function of the volume of titrant added. The shape of the titration curve makes it possible to determine the equivalence point.
Strong Acid–Strong Base Titrations
Are a type of titration used to determine the concentration of an acid or base in a solution. This type of titration involves the use of a strong acid and a strong base, and the endpoint is determined by the indicator used.
Weak Acid–Strong Base Titrations
Are a type of titration used to determine the concentration of an acid or base in a solution. In this type of titration, a strong base is added to a weak acid until the acid is completely neutralized. The amount of base added is then used to calculate the concentration of the acid. This type of titration Is useful for determining the concentration of weak acids, such as acetic acid, in a solution.
The titration curve for a weak acid–strong base titration differs from the curve for a strong acid–strong base titration in three noteworthy ways:
The solution of the weak acid has a higher initial pH than a solution of a strong acid of the same concentration.
The pH change in the rapid-rise portion of the curve near the equivalence point is smaller for the weak acid than for the strong acid.
The pH at the equivalence point is above 7.00 for the weak acid titration.
Titrating with an Acid–Base Indicator
An indicator is a compound that changes color in solution over a specific pH range. Optimally, an indicator should change color at the equivalence point in a titration.
The point in a titration where the indicator changes color is called the end point to distinguish it from the equivalence point that it closely approximates.
Solubility Equilibria
Solubility-product constant
The equilibrium constant indicates how soluble the solid is in water.
The coefficient for each ion in the equilibrium equation also equals its subscript in the compound’s chemical formula.
Molar solubility
Is the number of moles of solute that dissolve in forming 1 L of saturated solution of the solute (mol/L).
Factors That Affect Solubility
Solubility is affected by temperature and by the presence of other solutes. The presence of an acid, for example, can have a major influence on the solubility of a substance.
The Common-Ion Effect
Is a phenomenon in chemistry that occurs when a common ion is added to a solution.
This can cause the solubility of a compound to decrease, or the pH of the solution to change.
The Common-Ion Effect is important to consider when making solutions, as it can affect the concentration of the solution and the reaction rate of the compounds in the solution.
Solubility and pH
The solubility of almost any ionic compound is affected if the solution is made sufficiently acidic or basic. The effects are noticeable, however, only when one (or both) ions in the compound is at least moderately acidic or basic.
In general, the solubility of a compound containing a basic anion (that is, the anion of a weak acid) increases as the solution becomes more acidic.
Formation of Complex Ions
A characteristic property of metal ions is their ability to act as Lewis acids toward water molecules, which act as Lewis bases.Lewis bases other than water can also interact with metal ions, particularly transition-metal ions. Such interactions can dramatically affect the solubility of a metal salt.
Complex ion
A complex ion is a molecule or ion composed of two or more atoms held together by chemical bonds.Complex ions are formed when a metal atom or ion binds to one or more molecules or ions.
Formation constant

Amphoterism
Some metal oxides and hydroxides that are relatively insoluble in water dissolve in strongly acidic and strongly basic solutions. These substances, called amphoteric oxides and amphoteric hydroxides,are soluble in strong acids and bases because they themselves are capable of behaving as either an acid or base.
Precipitation and Separation of Ions
Equilibrium can be achieved starting with the substances on either side of a chemical equation.
Selective Precipitation of Ions
Is a process used to separate ions from a solution based on their solubility.
This process involves adding a reagent to the solution that will cause certain ions to precipitate out of the solution, while leaving other ions in the solution.
This process is often used in industrial processes to separate ions from a solution for further processing.
Qualitative Analysis for Metallic Elements
Qualitative analysis determines only the presence or absence of a particular metal ion relative to some threshold, whereas quantitative analysis determines how much of a given substance is present.
Typically, such analyses proceed in three stages:
(1) The ions are separated into broad groups on the basis of solubility properties.
(2) The ions in each group are separated by selectively dissolving members in the group.
(3) The ions are identified by means of specific tests.
 Knowt
Knowt