
Chapter 3 (Campbell's Biology in Focus)
Carbon Compounds Life
An organic compound is a compound containing carbon
On the molecular scale, members of three of these classes-- carbohydrates, proteins, and nucleic acids-- are huge and are therefore called macromolecules
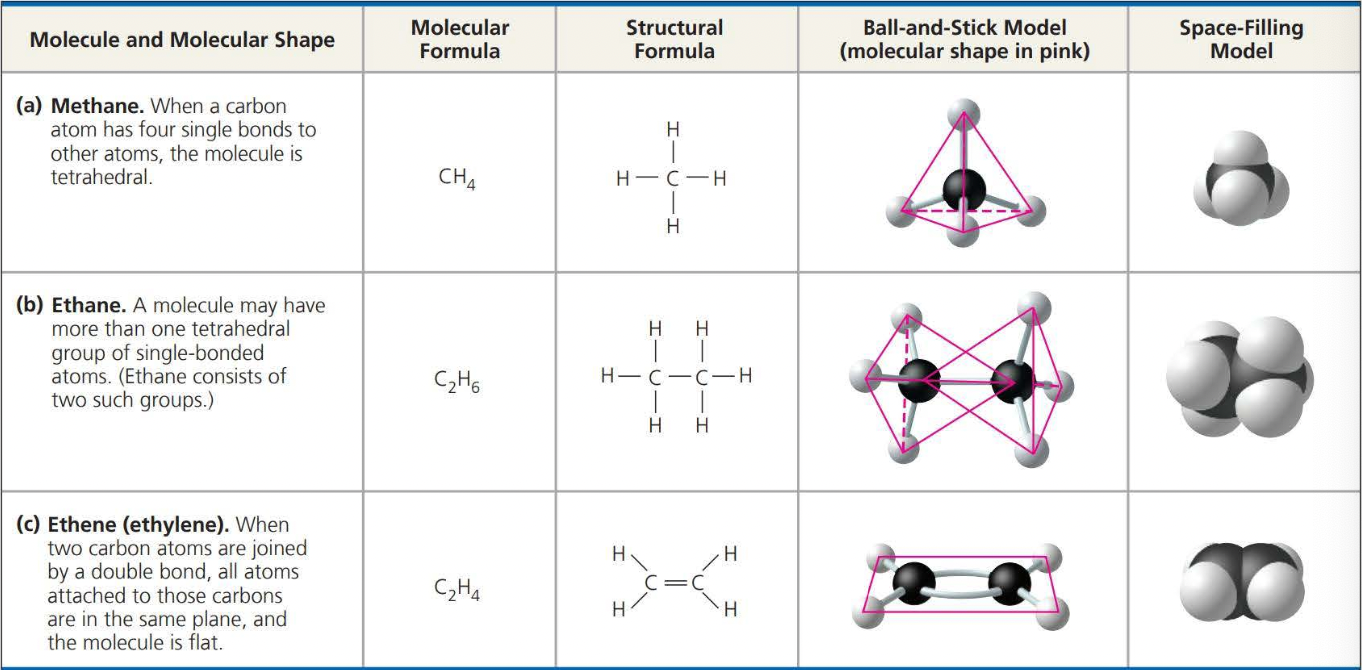
Molecules consisting of only carbon and hydrogen are called hydrocarbons
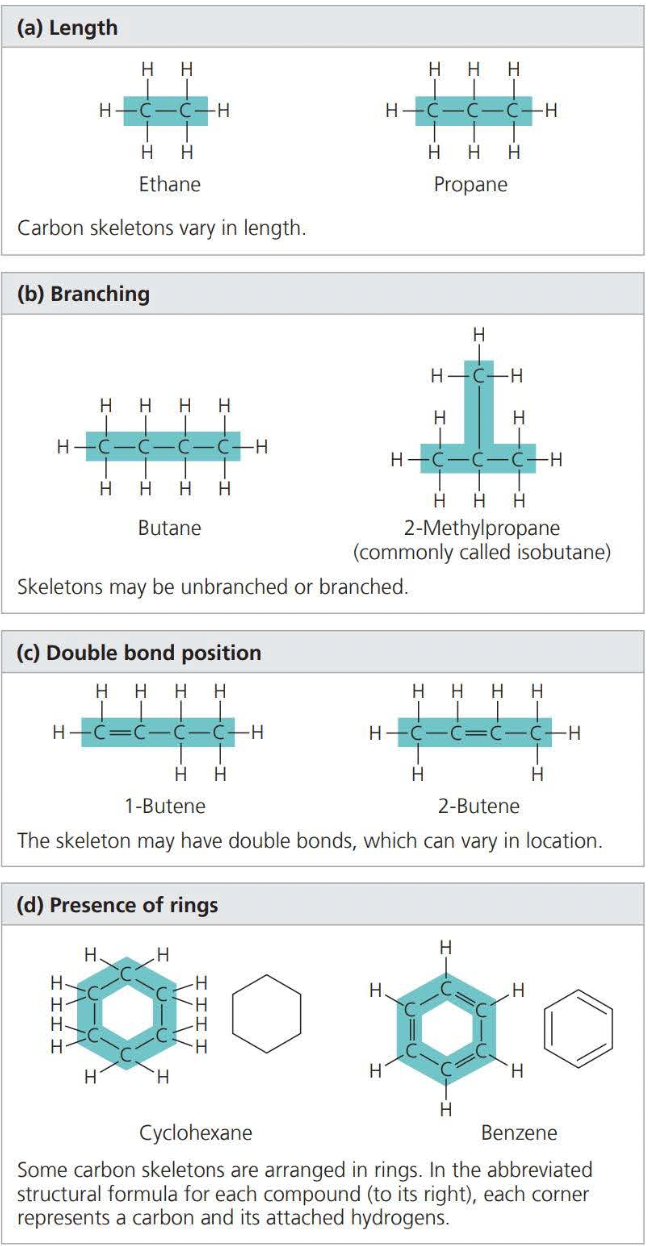
Isomers
Isomers are variations in the architecture of organic molecules
Structural isomers differ in the covalent arrangements of their atoms
In cis-trans isomers, carbons have covalent bonds to the same atoms, but their atoms differ in their spatial arrangements due to the inflexibility of double bonds
Enantiomers are isomers that are mirror images of each other and that differ in shape due to the presence of an asymmetric carbon, one that is attached to four different atoms or groups of atoms
Types of Chemical Groups:


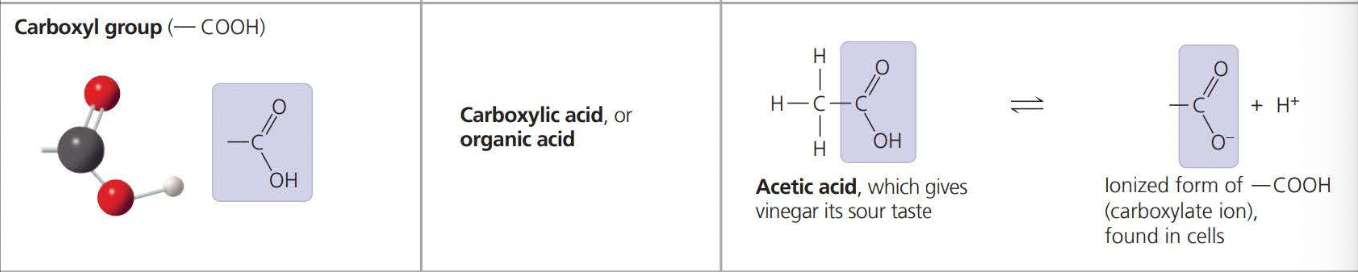
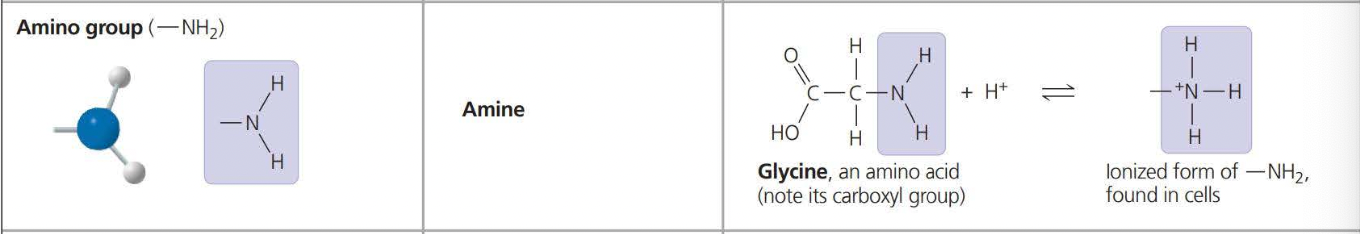

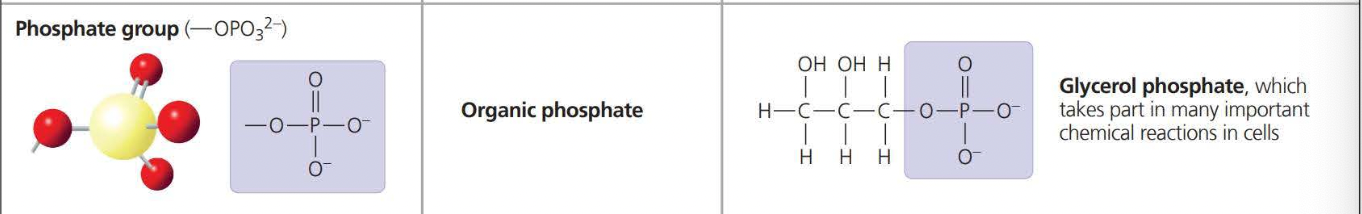

The above photos are known as Functional Groups. A functional group affects the molecular function by being directly involved in chemical reactions
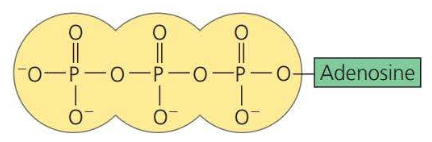
ATP
ATP is known as adenosine triphosphate, this is a more complicated organic phosphate
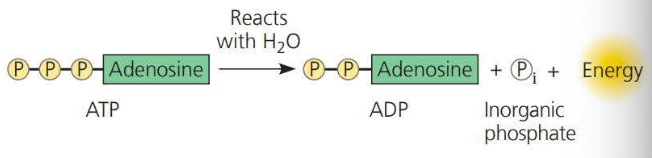
This is converter into ADP, known as Adenosine Diphosphate by way of hydration (adding water to a compound)
Macromolecules, Polymers, and Monomers
A polymer is a long molecule consisting of many similar or identical building blocks linked by covalent bonds
A monomer is the building block that creates a polymer
The chemical mechanism by which cells make polymers (polymerization) and break them down is similar for all classes of large biological molecules.
In cells, their processes are facilitated by enzymes.
Enzymes are specialized macromolecules(usually proteins) that speed up chemical reactions
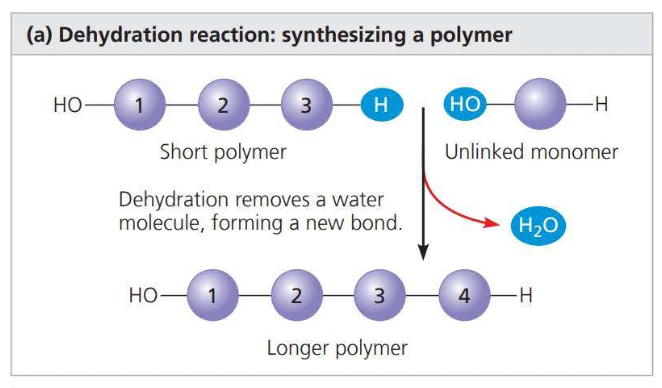
A dehydration reaction is when a water molecule is lost during a chemical reaction.
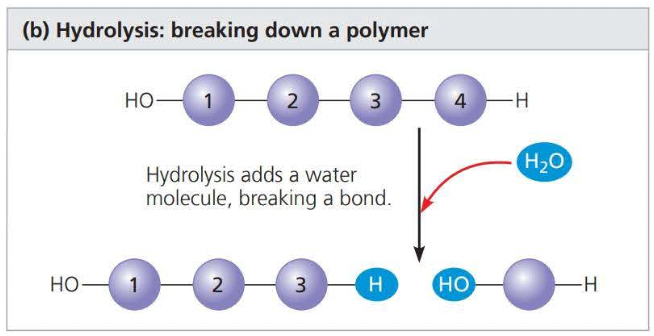
a hydrolysis reaction disassembles a polymer into monomers through hydrolysis
Carbohydrates serve as fuel and building material
Carbohydrates include both sugars and polymers of sugars.
The simplest carbohydrates are monosaccharides which are simple sugars
These are the monomers from which more complex carbohydrates are built.
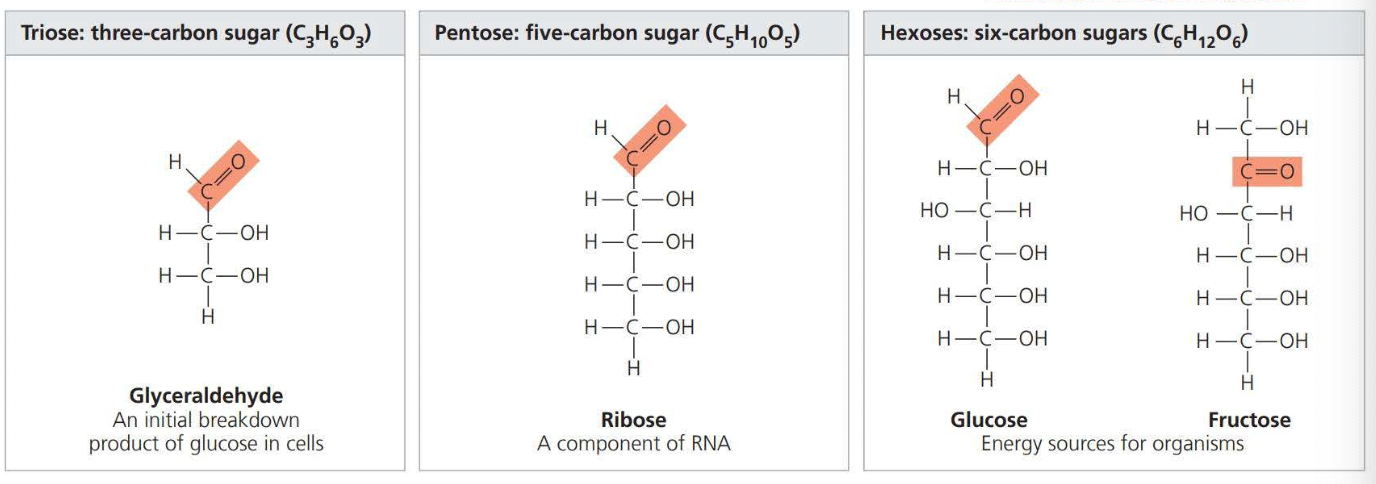
Sugars
Monosaccharides generally have molecular formulas that are some multiples of the unit CH2O.
Glucose (C6H12O6) is the most common monosaccharide.
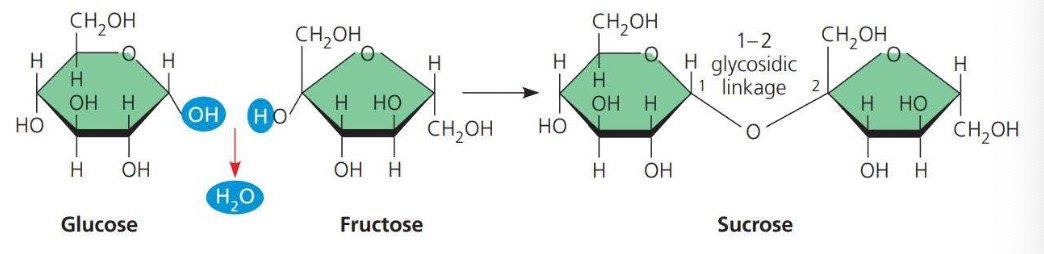
A disaccharide consists of two monosaccharides joined by a glycosidic linkage
A glycosidic linkage is a covalent bond formed between two monosaccharides
Polysaccharides are macromolecules, polymers with a few hundred to a few thousand monosaccharides joined by glycosidic linkages
Starch is stored in plant cells
Glycogen is stored in muscle cells
Structural cellulose fibers in plant cell walls are composed entirely of glucose monomers
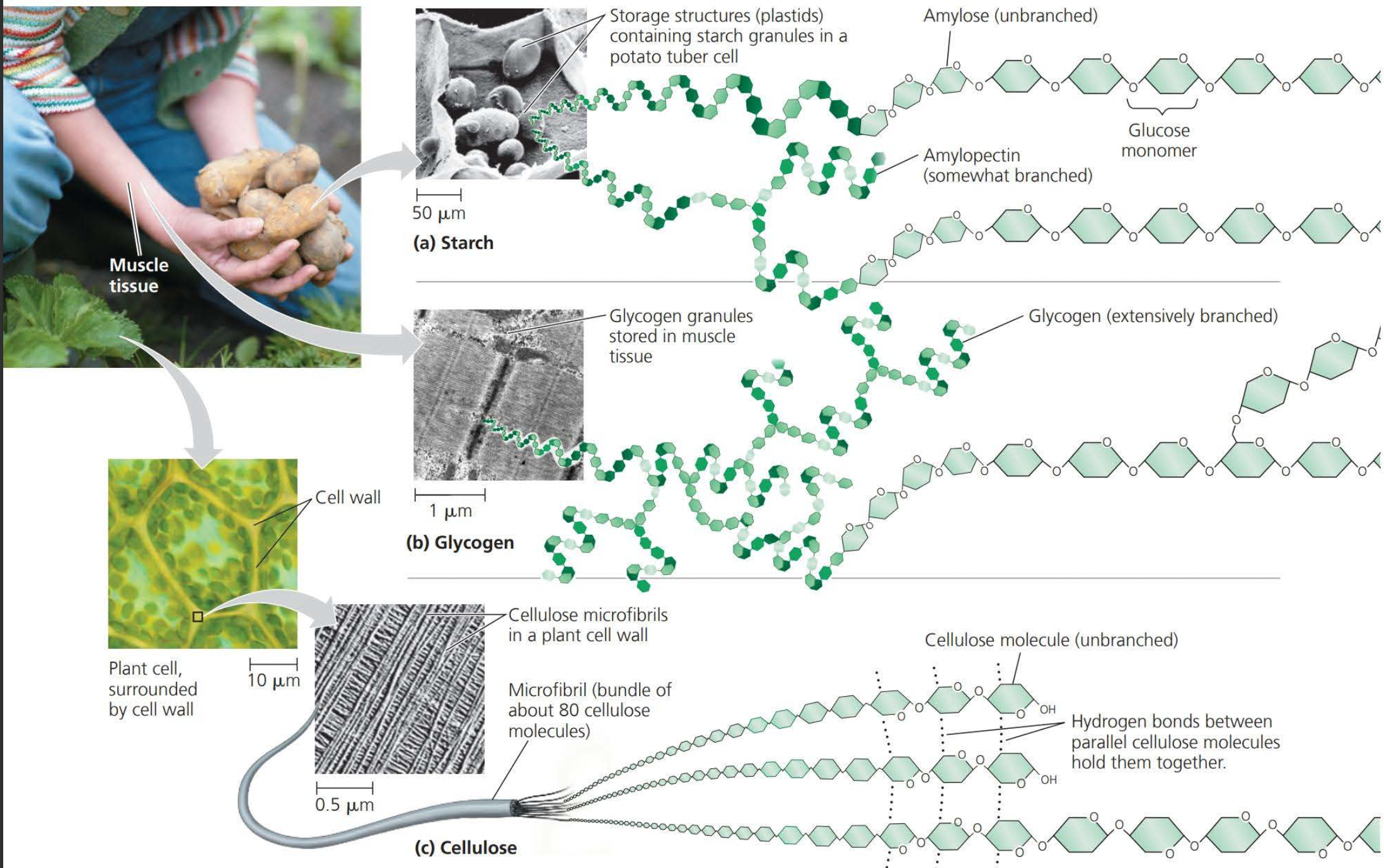
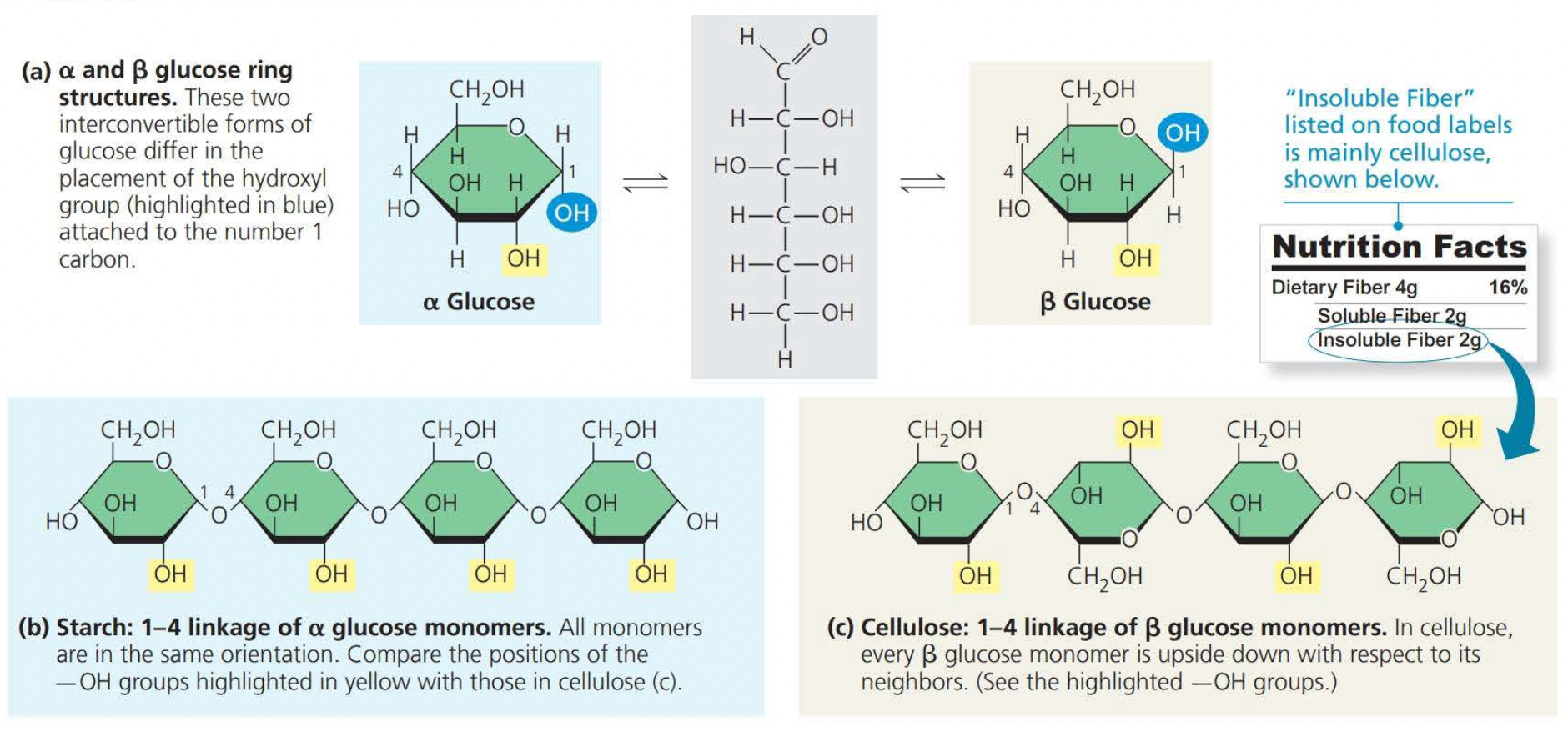
Note how in the above image these monomers are joined together by way of dehydration
Another important structural polysaccharide is chitin, this is used when arthropods build their exoskeleton
Lipids and Hydrophobic molecules
Compounds called lipids are grouped together because of their behaviour, they all mix poorly with water if at all. The hydrophobic behaviour of lipids is based on their molecular structure (these are nonpolar compounds.)
Lipids normally are hydrocarbons however some have polar bonds with oxygen
Fats are not polymers, they are large molecules assembled by dehydration reactions
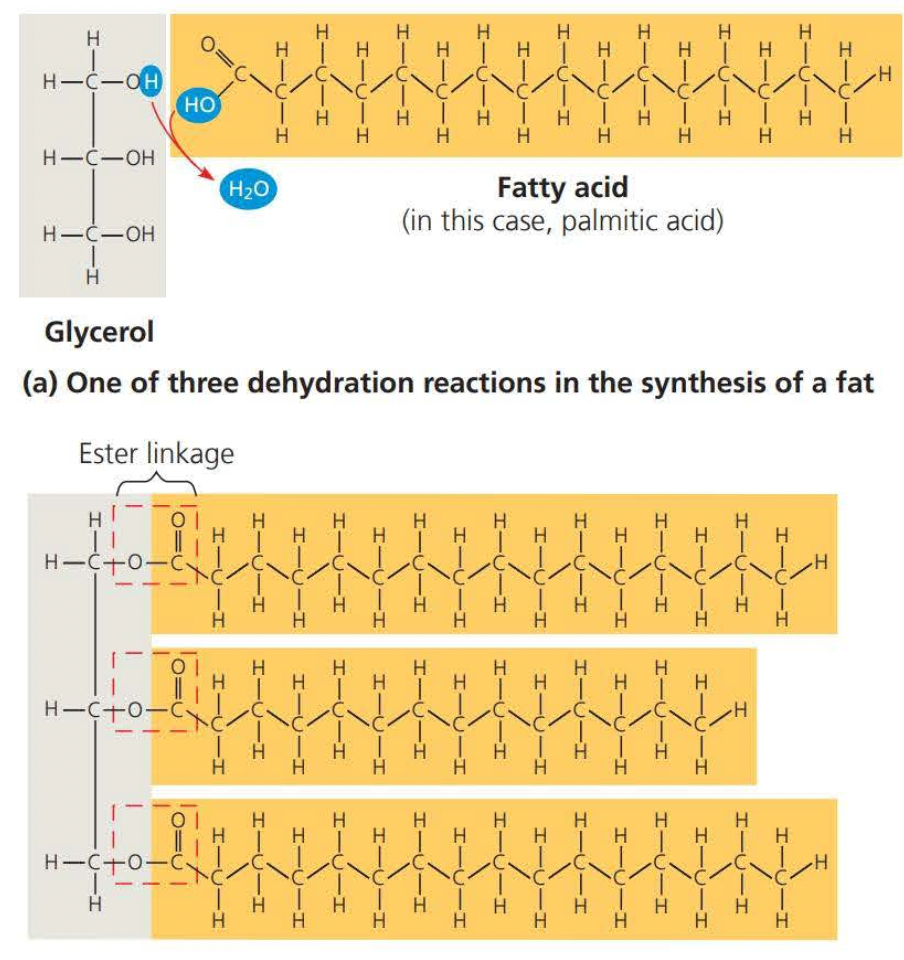
Fats consist of a glycerol molecule joined to three fatty acids
Glycerol is an alcohol and each of its three carbons bears a hydroxyl group
A fatty acid has a long carbon skeleton of usually 16-18 carbon atoms in length
the carbon at one end is a carboxyl group, the functional group that gives these molecules the name a fatty acid.
C-H bonds in a hydrocarbon atom are non-polar and are the reason these structures are hydrophobic
The link between glycerol and a fatty acid is called an ester linkage (it is formed through dehydration)
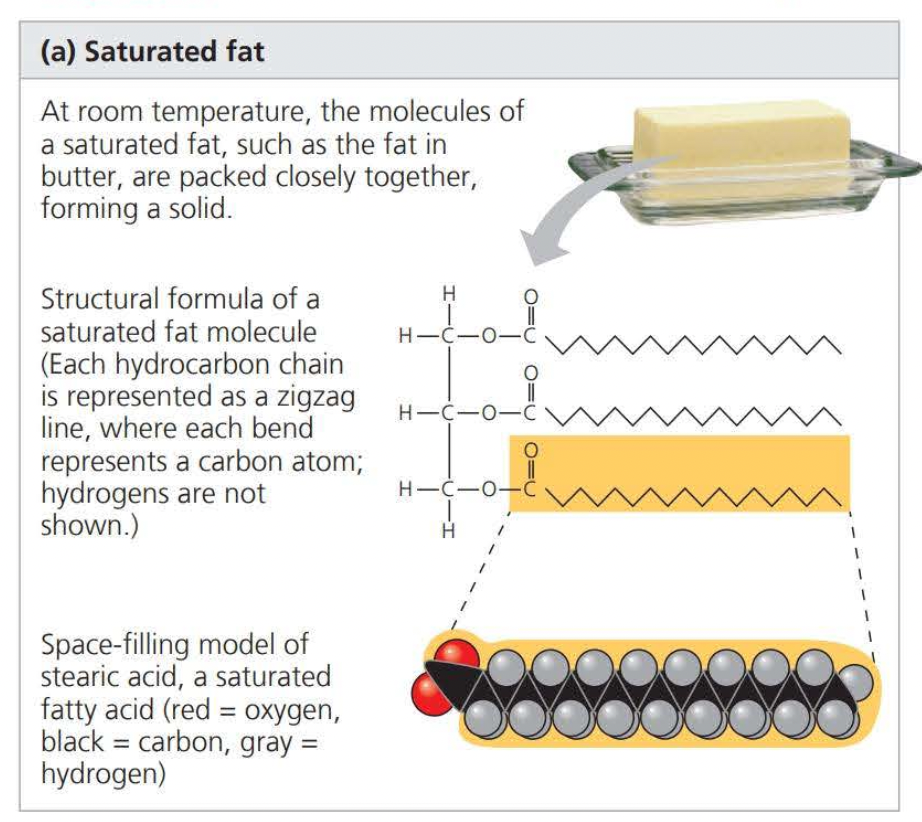
When there are no double bonds between carbons on a carbon skeleton the compound is said to be saturated
This results in a saturated fatty acid
An unsaturated compound is formed when there are double bonds between carbons
this results in an unsaturated fatty acid
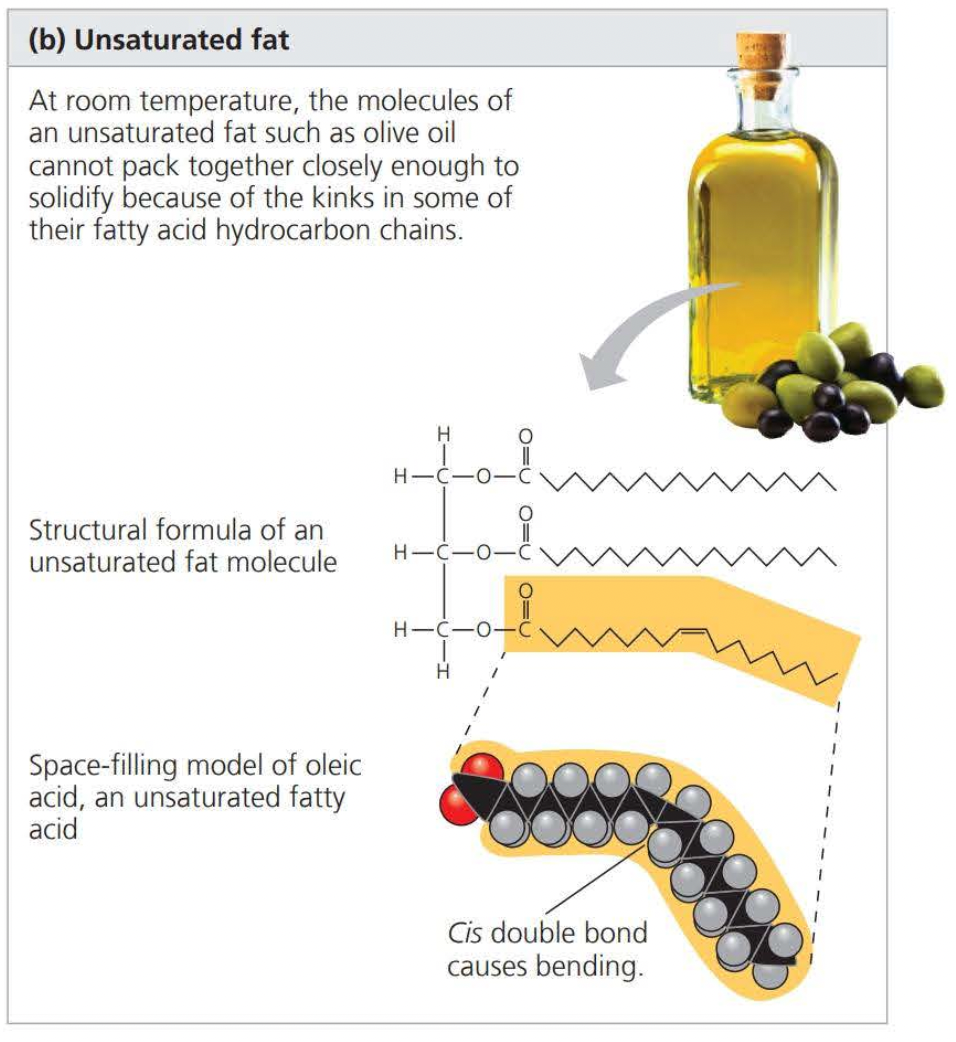
The phrase “hydrogen vegetable oils” on a food label means that unsaturated fats have been synthetically converted to saturated fats by adding a hydrogen atom, removing the double bond, and allowing it to solidify.
This process also produces unsaturated fats with trans double bonds, known as trans fats
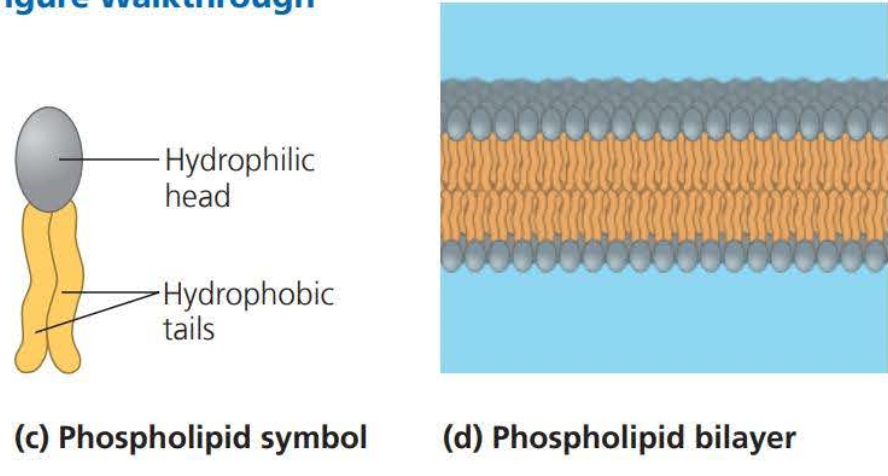
A phospholipid is similar to a fat molecule but only has two fatty acids attached to a glycerol molecule
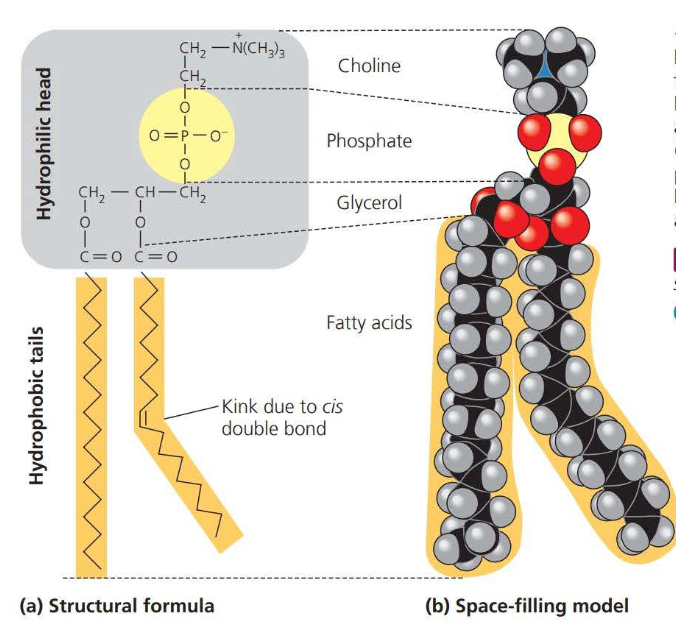
A phospholipid has a polar (hydrophilic) head and two nonpolar (hydrophobic) tails
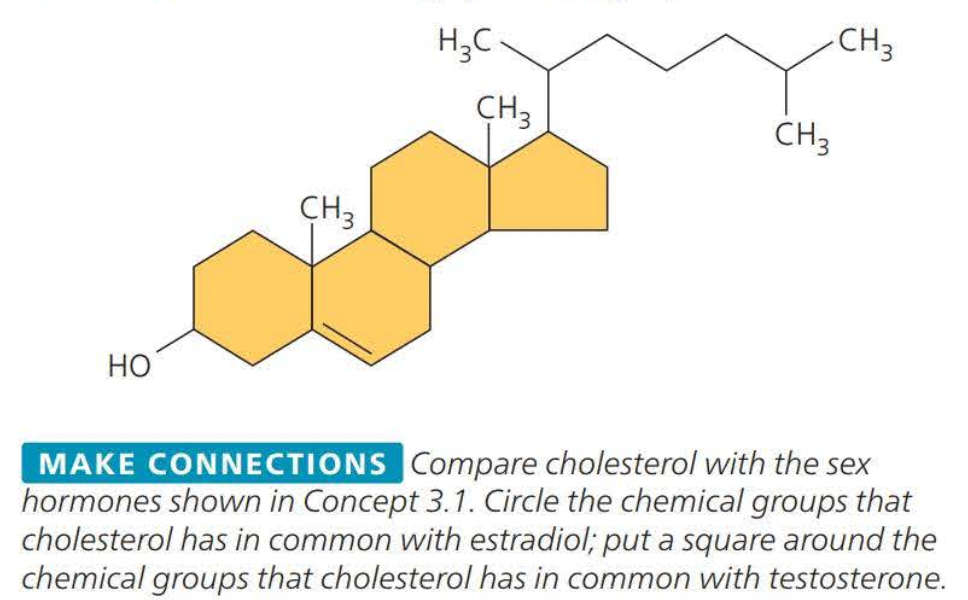
Steroids
Steroids are lipids characterized by a carbon skeleton consisting of four fused rings
Cholesterol is a crucial steroid stored in animals
cholesterol is a molecule from which other steroids including sex hormones, are synthesized.
Peptide bonds are bonds between amino acids
A polypeptide is a polymer of amino acids
A protein is a biologically functioning molecule made up of one or more polypeptides folded and failed in a specific three-dimensional shape
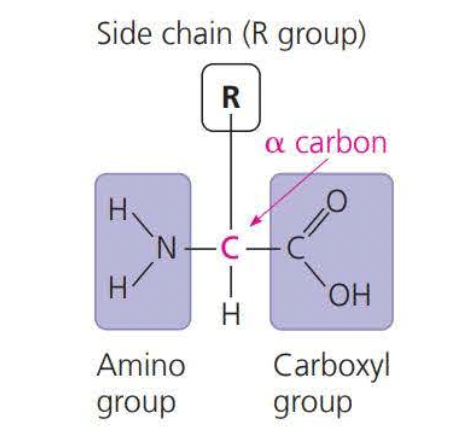
An amino acid is an organic molecule that both an amino group and a carboxyl group have
Enzymatic proteins - Selective acceleration of chemical reactions
Defensive proteins - Protection against disease
Storage proteins - Storage of amino acids
Transport proteins - Transport of substances
Hormonal proteins - Coordination of an organism's activities
Receptor proteins - Response of cell to chemical stimuli
Contractile and Motor proteins - Movement
Structural proteins - Support
There are a total of 20 amino acids making up proteins in the human body but there are over 500 amino acids
Because the side chains in amino acids are charged they are hydrophilic
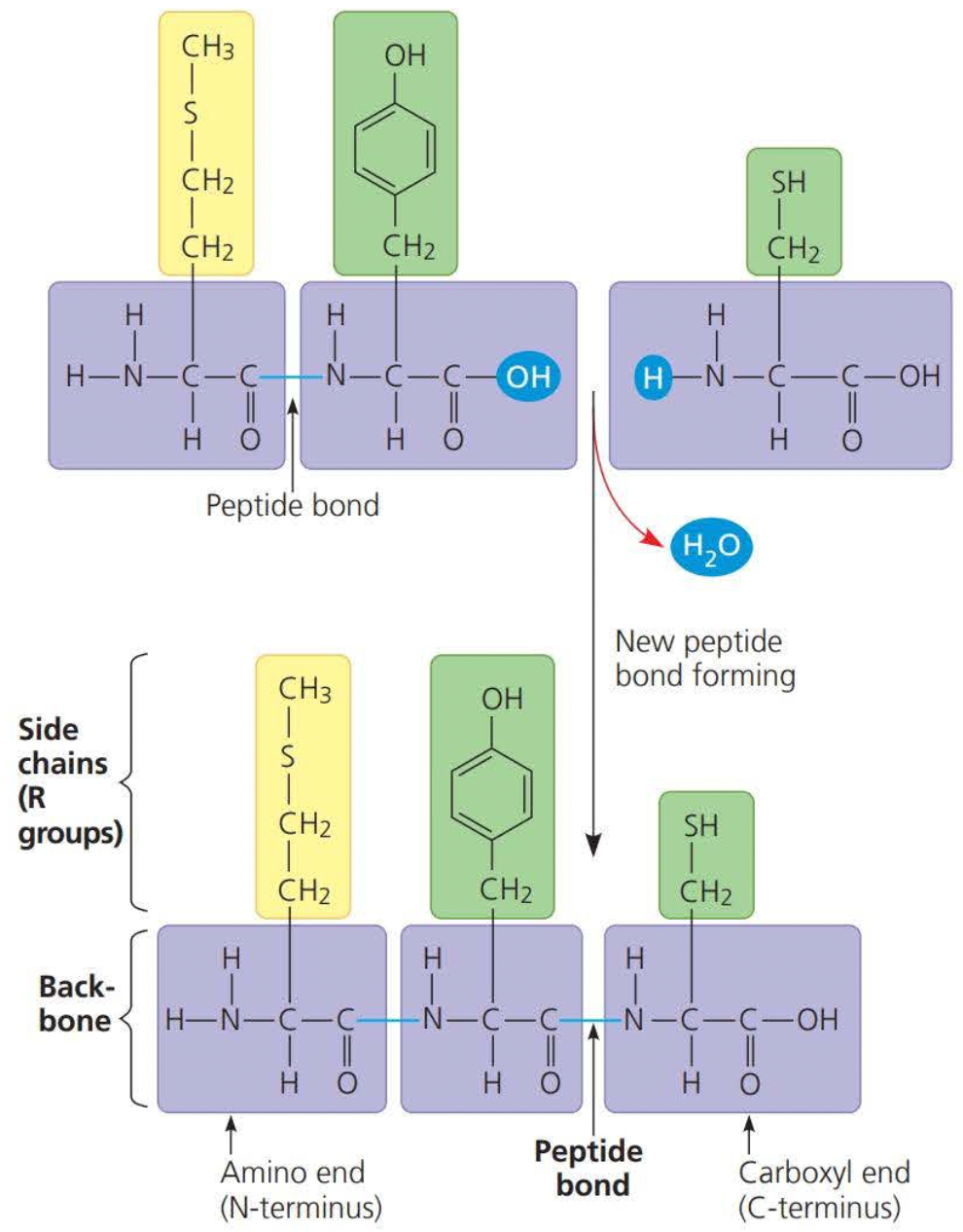
A polypeptide chain is formed through dehydration
this dehydration reaction forms a peptide bond
Levels of Proteins Structures
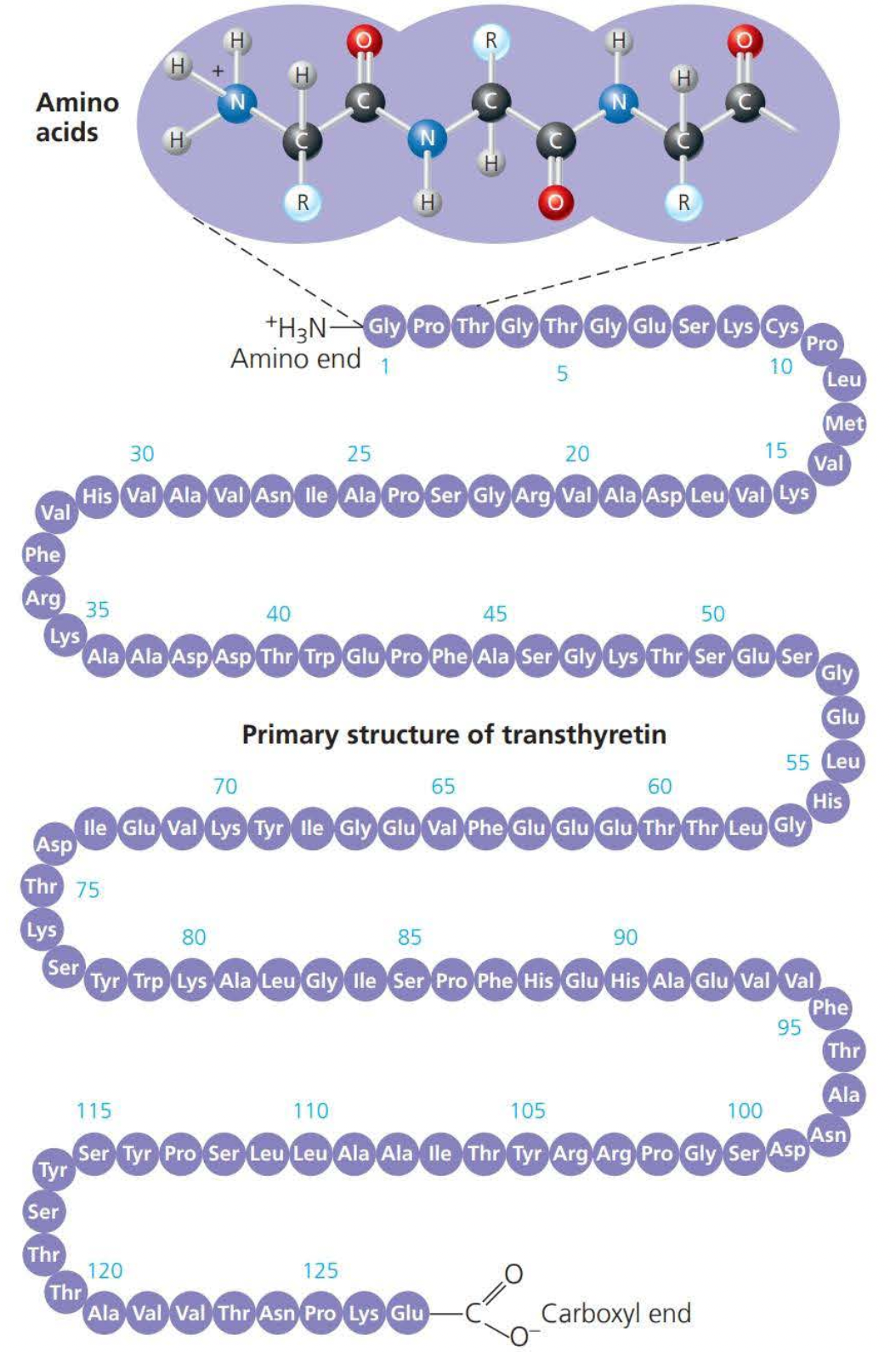
The primary structure of proteins is the order of its amino acids
Alpha helixes and Beta folds are known as the secondary structure
This is the result of hydrogen bonds between the repeating constituent of the polypeptide backbone
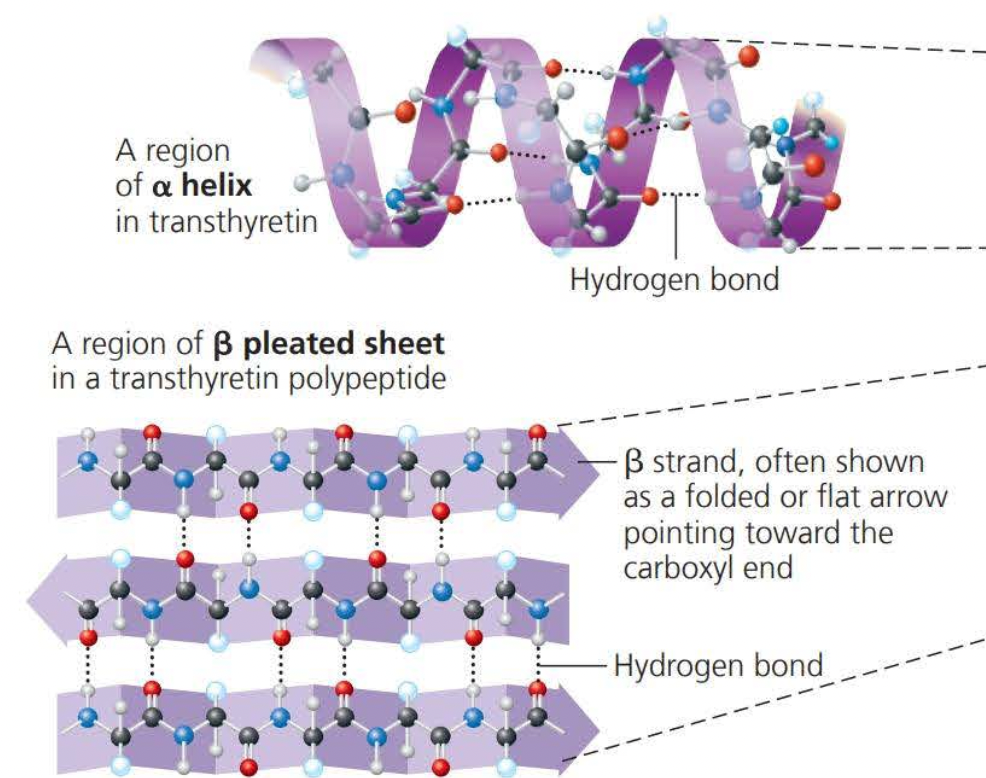
Secondary structures involve interactions between backbone constituents
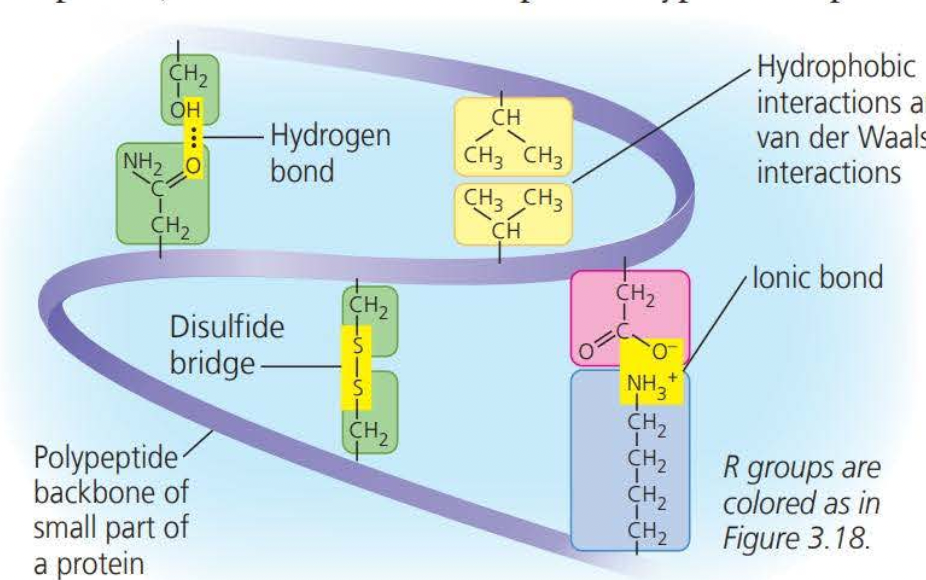
Tertiary structures are the overall shape of a polypeptide resulting from interactions of the side chains or the various amino acids.
One type of interaction that constitutes the shape of the tertiary structure is a hydrophobic interaction. As a polypeptide folds into its functional shape, amino acids with hydrophobic side chains (nonpolar) usually end up in clusters at the center of the protein due to contact with water
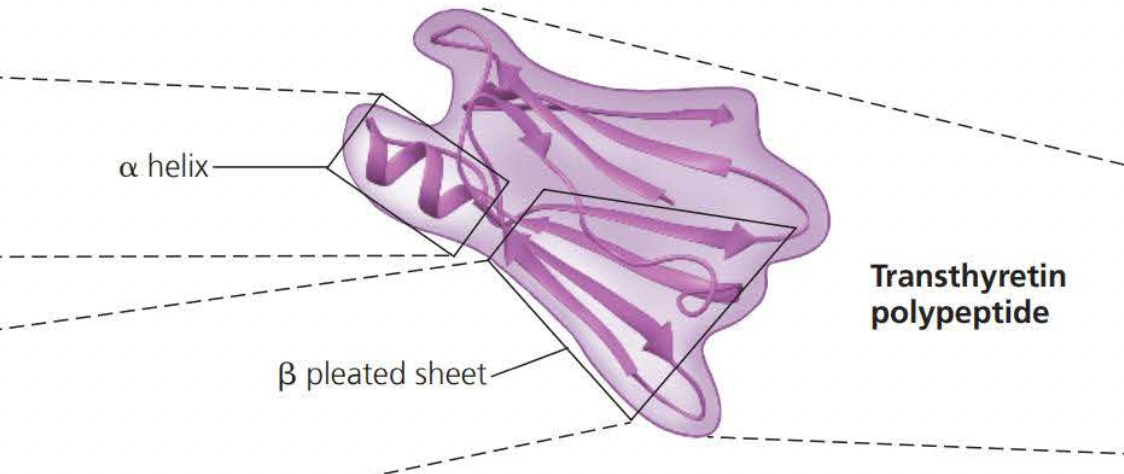
Covalent bonds called disulfide bridges further reinforce the shape of a protein
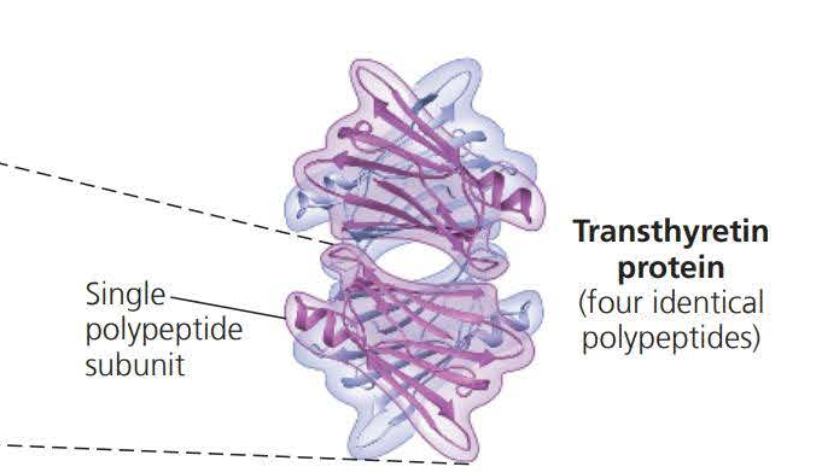
A quaternary structure is the overall protein structure that results from the aggression of these polypeptides subunits
Even a slight change in a primary structure can affect a protein’s structure and function
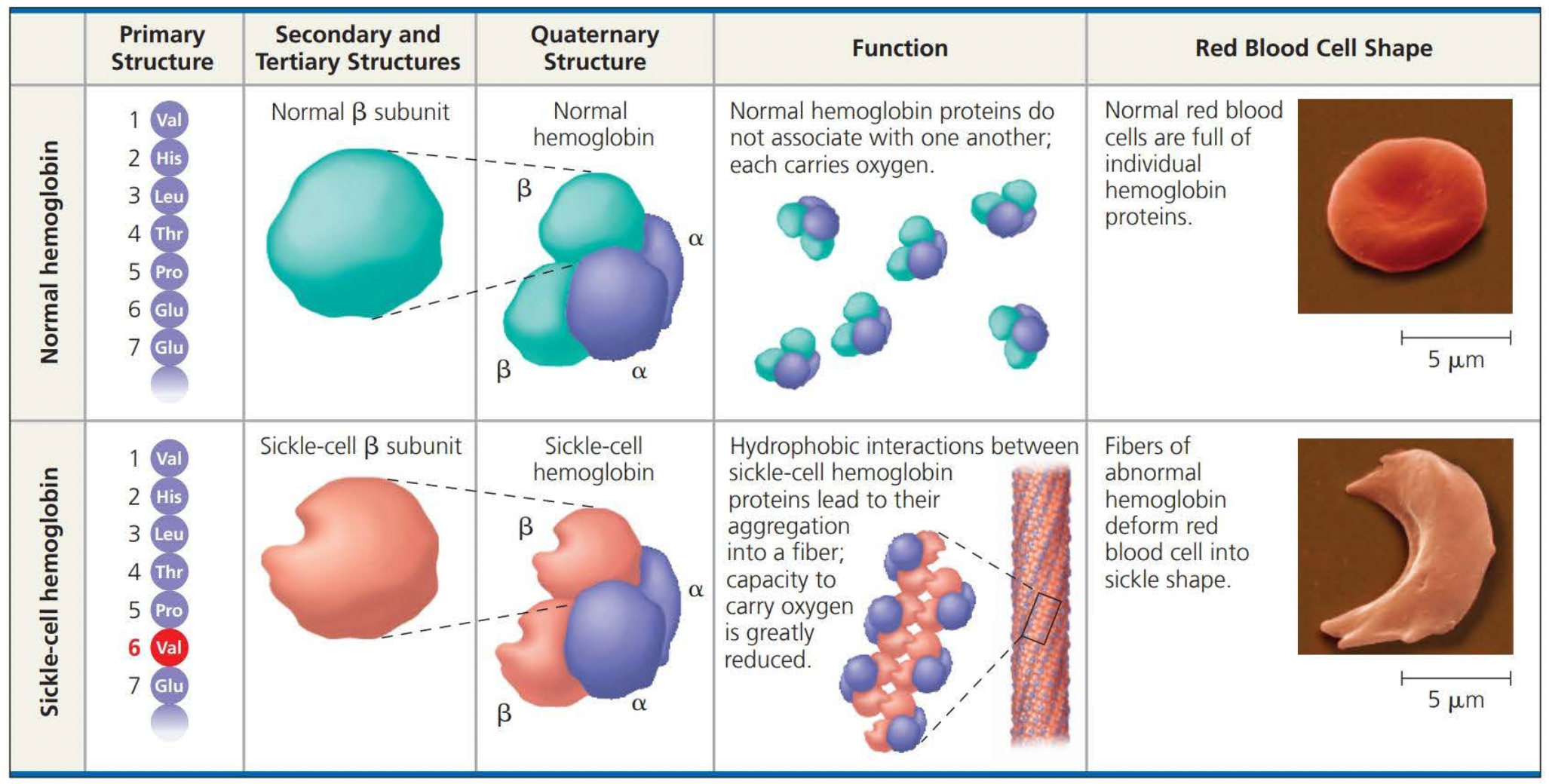
Things that denature proteins are
pH
Salt concentration
Temperature
Weak chemical bonds
Nucleic Acids
A gene is known as a discrete unit of inheritance that controls the amino acid sequence of a polypeptide
Nucleic acids are polymers made of monomers called nucleotides
DNA - Deoxyribolnucleic Acid
RNA - Ribonucleic Acid
DNA directs RNA synthesis and, through RNA, controls protein synthesis; this is known as gene expression
Nucleic acids are macromolecules that exist as polymers called polynucleotides
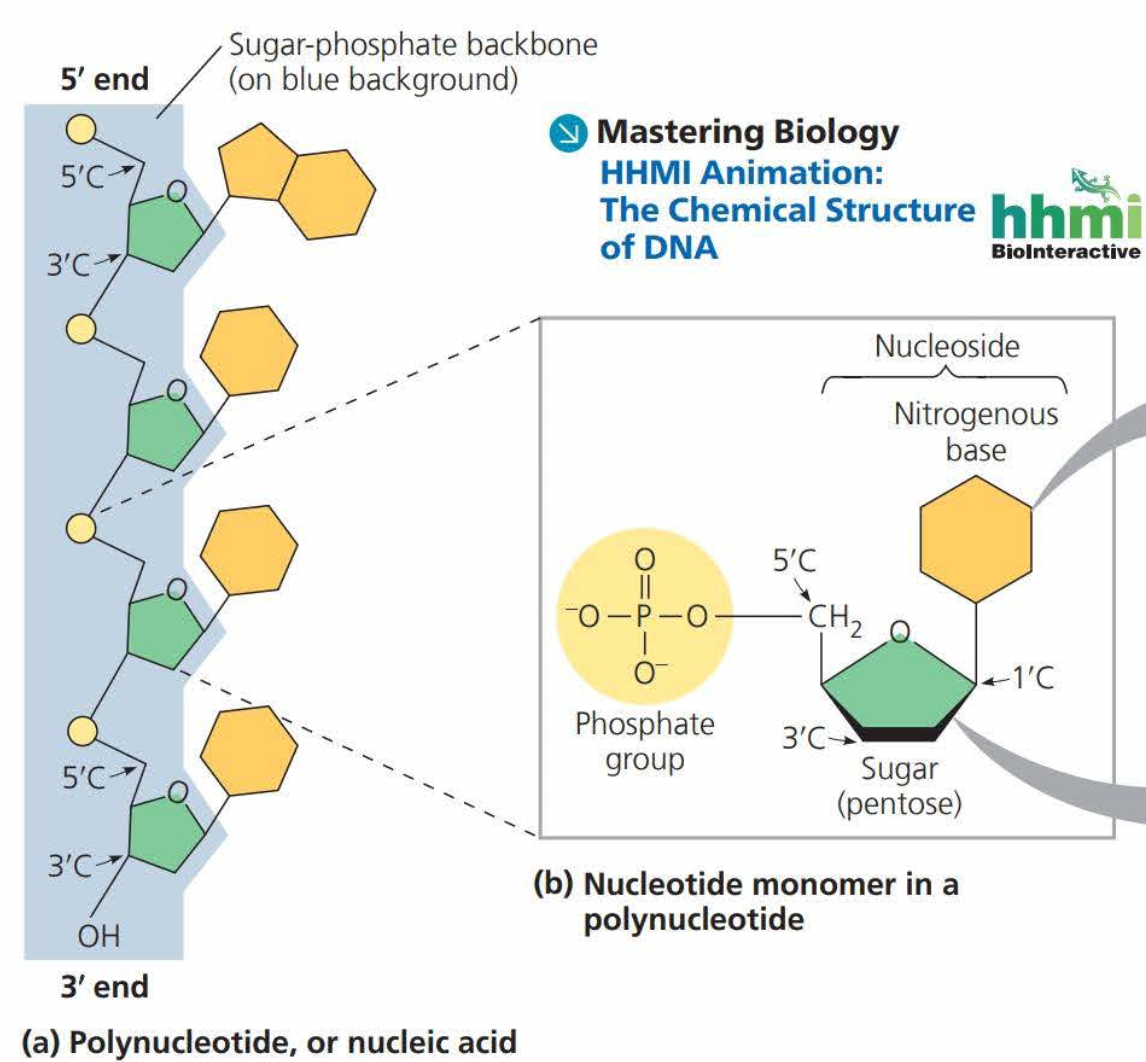
A portion of a nucleotide without a phosphate group is called a nucleoside
A pyrimidine has one-six membered ring of carbon and nitrogen atoms
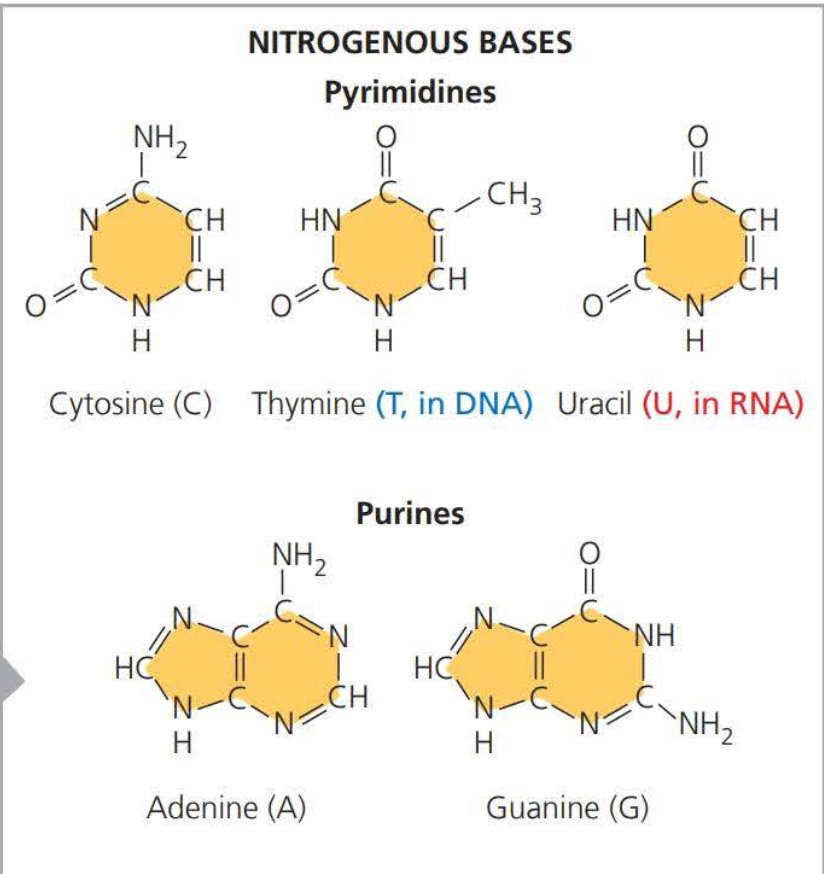
Purines are larger, with a six-membered ring fused to a five-membered
ring.
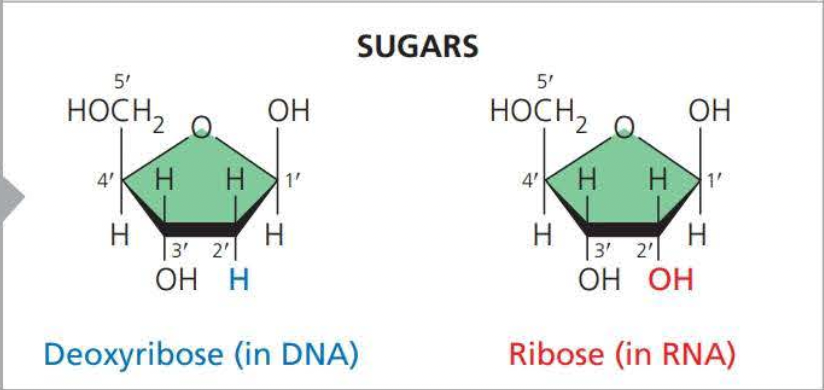
Two examples of sugars are
Deoxyribose (Adenine, Thymine, Cytosine, and Guanine )
Ribose (Adenine, Thymine, Cytosine, and Uracil)
DNA molecules have two polynucleotides or strands which wind around an imaginary axis forming a double helix
Two sugar-phosphate backbones run in opposite 5’ → 3’ directions from one another, this arrangement is known as antiparallel
Chapter 3 (Campbell's Biology in Focus)
Carbon Compounds Life
An organic compound is a compound containing carbon
On the molecular scale, members of three of these classes-- carbohydrates, proteins, and nucleic acids-- are huge and are therefore called macromolecules

Molecules consisting of only carbon and hydrogen are called hydrocarbons

Isomers
Isomers are variations in the architecture of organic molecules
Structural isomers differ in the covalent arrangements of their atoms
In cis-trans isomers, carbons have covalent bonds to the same atoms, but their atoms differ in their spatial arrangements due to the inflexibility of double bonds
Enantiomers are isomers that are mirror images of each other and that differ in shape due to the presence of an asymmetric carbon, one that is attached to four different atoms or groups of atoms
Types of Chemical Groups:







The above photos are known as Functional Groups. A functional group affects the molecular function by being directly involved in chemical reactions

ATP
ATP is known as adenosine triphosphate, this is a more complicated organic phosphate

This is converter into ADP, known as Adenosine Diphosphate by way of hydration (adding water to a compound)

Macromolecules, Polymers, and Monomers
A polymer is a long molecule consisting of many similar or identical building blocks linked by covalent bonds
A monomer is the building block that creates a polymer
The chemical mechanism by which cells make polymers (polymerization) and break them down is similar for all classes of large biological molecules.
In cells, their processes are facilitated by enzymes.
Enzymes are specialized macromolecules(usually proteins) that speed up chemical reactions
A dehydration reaction is when a water molecule is lost during a chemical reaction.

a hydrolysis reaction disassembles a polymer into monomers through hydrolysis

Carbohydrates serve as fuel and building material
Carbohydrates include both sugars and polymers of sugars.
The simplest carbohydrates are monosaccharides which are simple sugars
These are the monomers from which more complex carbohydrates are built.

Sugars
Monosaccharides generally have molecular formulas that are some multiples of the unit CH2O.
Glucose (C6H12O6) is the most common monosaccharide.
A disaccharide consists of two monosaccharides joined by a glycosidic linkage
A glycosidic linkage is a covalent bond formed between two monosaccharides

Polysaccharides are macromolecules, polymers with a few hundred to a few thousand monosaccharides joined by glycosidic linkages
Starch is stored in plant cells
Glycogen is stored in muscle cells
Structural cellulose fibers in plant cell walls are composed entirely of glucose monomers


Note how in the above image these monomers are joined together by way of dehydration
Another important structural polysaccharide is chitin, this is used when arthropods build their exoskeleton
Lipids and Hydrophobic molecules
Compounds called lipids are grouped together because of their behaviour, they all mix poorly with water if at all. The hydrophobic behaviour of lipids is based on their molecular structure (these are nonpolar compounds.)
Lipids normally are hydrocarbons however some have polar bonds with oxygen
Fats are not polymers, they are large molecules assembled by dehydration reactions
Fats consist of a glycerol molecule joined to three fatty acids
Glycerol is an alcohol and each of its three carbons bears a hydroxyl group
A fatty acid has a long carbon skeleton of usually 16-18 carbon atoms in length
the carbon at one end is a carboxyl group, the functional group that gives these molecules the name a fatty acid.
C-H bonds in a hydrocarbon atom are non-polar and are the reason these structures are hydrophobic
The link between glycerol and a fatty acid is called an ester linkage (it is formed through dehydration)

When there are no double bonds between carbons on a carbon skeleton the compound is said to be saturated
This results in a saturated fatty acid

An unsaturated compound is formed when there are double bonds between carbons
this results in an unsaturated fatty acid

The phrase “hydrogen vegetable oils” on a food label means that unsaturated fats have been synthetically converted to saturated fats by adding a hydrogen atom, removing the double bond, and allowing it to solidify.
This process also produces unsaturated fats with trans double bonds, known as trans fats
A phospholipid is similar to a fat molecule but only has two fatty acids attached to a glycerol molecule

A phospholipid has a polar (hydrophilic) head and two nonpolar (hydrophobic) tails

Steroids
Steroids are lipids characterized by a carbon skeleton consisting of four fused rings
Cholesterol is a crucial steroid stored in animals
cholesterol is a molecule from which other steroids including sex hormones, are synthesized.

Peptide bonds are bonds between amino acids
A polypeptide is a polymer of amino acids
A protein is a biologically functioning molecule made up of one or more polypeptides folded and failed in a specific three-dimensional shape
An amino acid is an organic molecule that both an amino group and a carboxyl group have

Enzymatic proteins - Selective acceleration of chemical reactions
Defensive proteins - Protection against disease
Storage proteins - Storage of amino acids
Transport proteins - Transport of substances
Hormonal proteins - Coordination of an organism's activities
Receptor proteins - Response of cell to chemical stimuli
Contractile and Motor proteins - Movement
Structural proteins - Support
There are a total of 20 amino acids making up proteins in the human body but there are over 500 amino acids
Because the side chains in amino acids are charged they are hydrophilic
A polypeptide chain is formed through dehydration
this dehydration reaction forms a peptide bond

Levels of Proteins Structures
The primary structure of proteins is the order of its amino acids

Alpha helixes and Beta folds are known as the secondary structure
This is the result of hydrogen bonds between the repeating constituent of the polypeptide backbone

Secondary structures involve interactions between backbone constituents
Tertiary structures are the overall shape of a polypeptide resulting from interactions of the side chains or the various amino acids.
One type of interaction that constitutes the shape of the tertiary structure is a hydrophobic interaction. As a polypeptide folds into its functional shape, amino acids with hydrophobic side chains (nonpolar) usually end up in clusters at the center of the protein due to contact with water

Covalent bonds called disulfide bridges further reinforce the shape of a protein

A quaternary structure is the overall protein structure that results from the aggression of these polypeptides subunits

Even a slight change in a primary structure can affect a protein’s structure and function

Things that denature proteins are
pH
Salt concentration
Temperature
Weak chemical bonds
Nucleic Acids
A gene is known as a discrete unit of inheritance that controls the amino acid sequence of a polypeptide
Nucleic acids are polymers made of monomers called nucleotides
DNA - Deoxyribolnucleic Acid
RNA - Ribonucleic Acid
DNA directs RNA synthesis and, through RNA, controls protein synthesis; this is known as gene expression
Nucleic acids are macromolecules that exist as polymers called polynucleotides

A portion of a nucleotide without a phosphate group is called a nucleoside
A pyrimidine has one-six membered ring of carbon and nitrogen atoms

Purines are larger, with a six-membered ring fused to a five-membered
ring.
Two examples of sugars are
Deoxyribose (Adenine, Thymine, Cytosine, and Guanine )
Ribose (Adenine, Thymine, Cytosine, and Uracil)

DNA molecules have two polynucleotides or strands which wind around an imaginary axis forming a double helix
Two sugar-phosphate backbones run in opposite 5’ → 3’ directions from one another, this arrangement is known as antiparallel
 Knowt
Knowt