
Chapter 10: Acids and Bases and Equilibrium
10.1: Acids and Bases
Acids
Arrhenius Acids: Substances that produce hydrogen ions when they dissolve in water.
Because acids produce ions in water, they are also electrolytes.
Svante Arrhenius — A Swedish chemist to first describe acids.
Naming Acids
When an acid dissolves in water to produce a hydrogen ion and a simple nonmetal anion, the prefix hydro– is used before the name of the nonmetal, and its –ide ending is changed to –ic acid.
Ex.: Hydrochloric acid, hydrobromic acid and hydrocyanic acid
The most common form of an oxygen-containing acid has a name that ends with –ic acid.
The name of its polyatomic anion ends in –ate.
Ex.: Sulfate, carbonate, acetate and phosphate
An acid that contains one less oxygen atom than the common form is named as an –ous acid.
Ex.: Phosphorous acid, sulfurous acid, and chlorous acid.
The name of its polyatomic anion ends with –ite.
Ex.: Sulfite, nitrite, phosphite, and chlorite.
Bases
Arrhenius Bases: These are ionic compounds that dissociate into metal ions and hydroxide ions when they dissolve in water. These bases are considered electrolytes as well.
Most Arrhhenius bases are formed from Group 1A and Group 2A metals.
Naming Bases
Typical Arrhenius bases are named as hydroxides.
Lithium Hydroxide
Sodium Hydroxide
Potassium Hydroxide
Calcium Hydroxide
Aluminum Hydroxide
Brønsted–Lowry Acids and Bases
J. N. Brønsted and T. M. Lowry — They expanded the definition of acids and bases in 1923.
Brønsted–Lowry acid: It can donate a hydrogen ion to another substance.
Brønsted–Lowry base: It can accept hydrogen ion.
Conjugate Acid–Base Pairs
According to the Brønsted–Lowry theory, a conjugate acid–base pair consists of molecules or ions related by the loss of one H+ by an acid, and the gain of one H+ by a base.
Amphoteric: Substances that can act as acids and bases.
10.2: Strengths of Acids and Bases
The strength of an acid is determined by the moles of H3O+ that are produced for each mole of acid that dissolves.
The strength of a base is determined by the moles of OH- that are produced for each mole of base that dissolves.
Strong acids: These are examples of strong electrolytes because they donate H+ so easily that their ionization in water is virtually complete.
Weak Acids: These are weak electrolytes because they ionize slightly in water, which produces only a few ions.
Diprotic Acid: Carbonic acid that has two H+, which ionize one at a time
Strong Bases: These are ionic compounds that dissociate in water to give an aqueous solution of metal ions and hydroxide ions.
Weak Bases: These are weak electrolytes that are poor acceptors of hydrogen ions and produce very few ions in solution.
10.3: Acid-Base Equilibrium
Equilibrium: The rates of the forward and reverse reactions become equal.
The reactants form products at the same rate that the products form reactants.
It has been reached when no further change takes place in the concentrations of the reactants and products.
Le Châtelier’s principle: It states that when equilibrium is disturbed, the rates of the forward and reverse reactions change to relieve that stress and reestablish equilibrium.
10.4: Ionization of Water
In pure water, a few water molecules transfer H+ to other water molecules, producing small, but equal, amounts of H3O+ and OH-.
In pure water, the molar concentrations of H3O+ and OH- are each 1.0 x 10^-7 M.
The ion product constant for water:
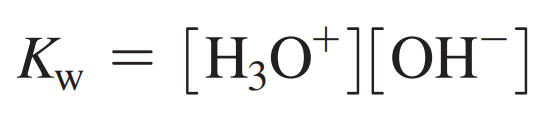
In acidic solutions, the [H3O+] is greater than the [OH-].
In basic solutions, the [OH-] is greater than the [H3O+].
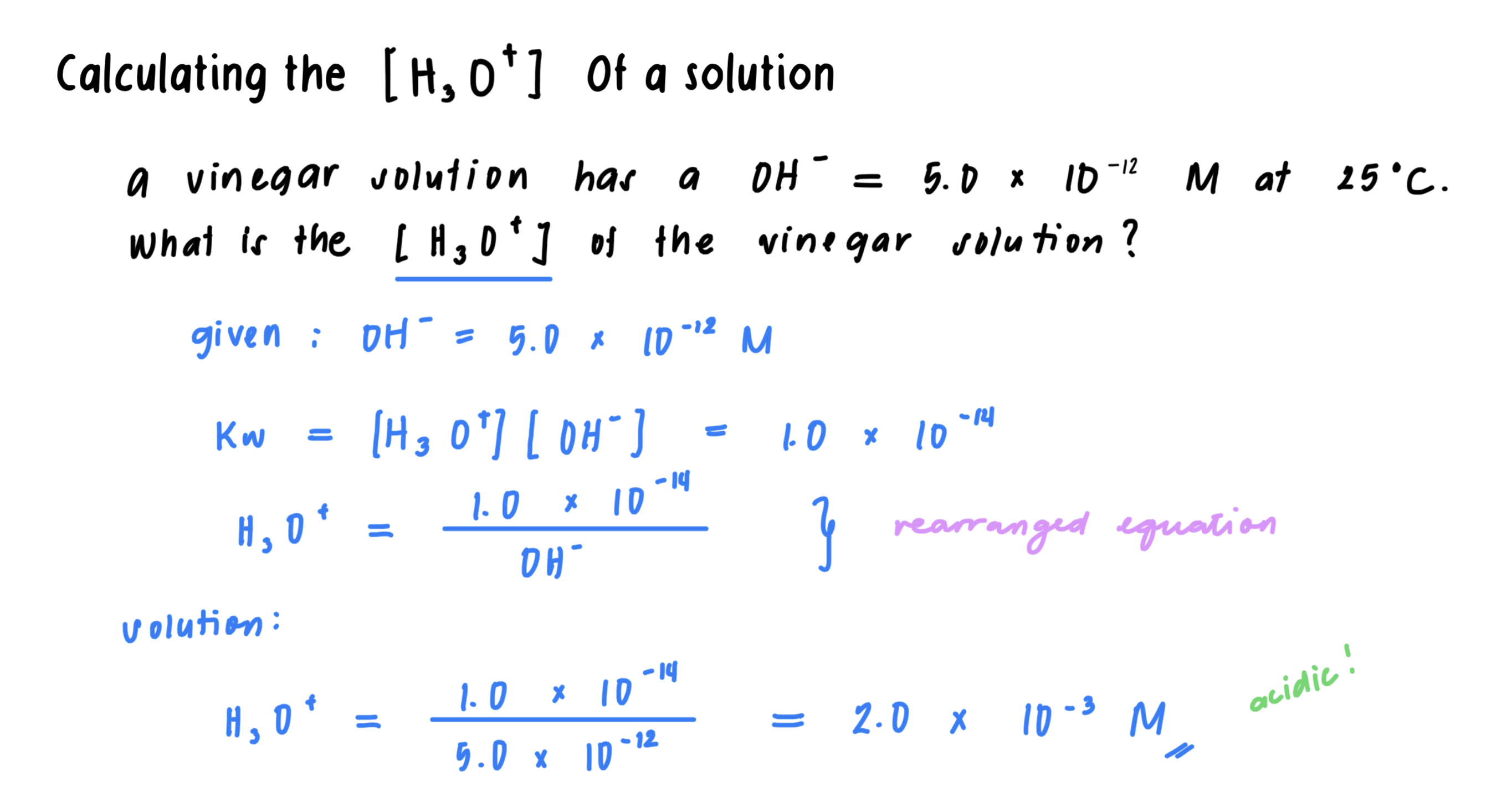
10.5: The pH Scale
pH Scale: A range of numbers typically from 0 to 14, which represents the [H3O+] of the solution.
A neutral solution has a pH of 7.0.
In acidic solutions, the pH is below 7.0.
In basic solutions, the pH is above 7.0.
Mathematically, pH is the negative logarithm of the hydronium ion concentration, pH = –log [H3O+].
10.6: Reactions of Acid and Bases
Salt: An ionic compound that does not have H+ as the cation or OH- as the anion.
Acids react with certain metals to produce hydrogen gas and salt.
Metals that react with acids include potassium, sodium, calcium, magnesium, aluminum, zinc, iron, and tin.
When an acid is added to a carbonate or bicarbonate, the products are carbon dioxide gas, water, and an ionic compound.
Neutralization: A reaction between an acid and a base to produce water and salt.
Titration: A laboratory procedure in which we neutralize an acid sample with a known amount of base.
In the titration, we neutralize the acid by adding a volume of base that contains a matching number of moles of OH-.
We know that neutralization has taken place when the phenolphthalein in the solution changes from colorless to pink. This is called the neutralization endpoint.
From the volume added and molarity of the NaOH solution, we can calculate the number of moles of NaOH, the moles of acid, and then the concentration of the acid.

Antacids: These are substances used to neutralize excess stomach acid.
10.7: Buffers
Buffer Solution: It maintains pH by neutralizing small amounts of added acid or base.
A buffer contains either a weak acid and its salt or a weak base and its salt.
When an acid or base is added to a buffer solution, there is little change in pH.
Acidosis: A condition that occurs when there’s an increase in the CO2 level that leads to a low blood pH concentration.
Alkalosis: A condition that occurs when there’s a decrease in the CO2 level that leads to a high blood pH concentration.
Chapter 10: Acids and Bases and Equilibrium
10.1: Acids and Bases
Acids
Arrhenius Acids: Substances that produce hydrogen ions when they dissolve in water.
Because acids produce ions in water, they are also electrolytes.
Svante Arrhenius — A Swedish chemist to first describe acids.
Naming Acids
When an acid dissolves in water to produce a hydrogen ion and a simple nonmetal anion, the prefix hydro– is used before the name of the nonmetal, and its –ide ending is changed to –ic acid.
Ex.: Hydrochloric acid, hydrobromic acid and hydrocyanic acid
The most common form of an oxygen-containing acid has a name that ends with –ic acid.
The name of its polyatomic anion ends in –ate.
Ex.: Sulfate, carbonate, acetate and phosphate
An acid that contains one less oxygen atom than the common form is named as an –ous acid.
Ex.: Phosphorous acid, sulfurous acid, and chlorous acid.
The name of its polyatomic anion ends with –ite.
Ex.: Sulfite, nitrite, phosphite, and chlorite.
Bases
Arrhenius Bases: These are ionic compounds that dissociate into metal ions and hydroxide ions when they dissolve in water. These bases are considered electrolytes as well.
Most Arrhhenius bases are formed from Group 1A and Group 2A metals.
Naming Bases
Typical Arrhenius bases are named as hydroxides.
Lithium Hydroxide
Sodium Hydroxide
Potassium Hydroxide
Calcium Hydroxide
Aluminum Hydroxide
Brønsted–Lowry Acids and Bases
J. N. Brønsted and T. M. Lowry — They expanded the definition of acids and bases in 1923.
Brønsted–Lowry acid: It can donate a hydrogen ion to another substance.
Brønsted–Lowry base: It can accept hydrogen ion.
Conjugate Acid–Base Pairs
According to the Brønsted–Lowry theory, a conjugate acid–base pair consists of molecules or ions related by the loss of one H+ by an acid, and the gain of one H+ by a base.
Amphoteric: Substances that can act as acids and bases.
10.2: Strengths of Acids and Bases
The strength of an acid is determined by the moles of H3O+ that are produced for each mole of acid that dissolves.
The strength of a base is determined by the moles of OH- that are produced for each mole of base that dissolves.
Strong acids: These are examples of strong electrolytes because they donate H+ so easily that their ionization in water is virtually complete.
Weak Acids: These are weak electrolytes because they ionize slightly in water, which produces only a few ions.
Diprotic Acid: Carbonic acid that has two H+, which ionize one at a time
Strong Bases: These are ionic compounds that dissociate in water to give an aqueous solution of metal ions and hydroxide ions.
Weak Bases: These are weak electrolytes that are poor acceptors of hydrogen ions and produce very few ions in solution.
10.3: Acid-Base Equilibrium
Equilibrium: The rates of the forward and reverse reactions become equal.
The reactants form products at the same rate that the products form reactants.
It has been reached when no further change takes place in the concentrations of the reactants and products.
Le Châtelier’s principle: It states that when equilibrium is disturbed, the rates of the forward and reverse reactions change to relieve that stress and reestablish equilibrium.
10.4: Ionization of Water
In pure water, a few water molecules transfer H+ to other water molecules, producing small, but equal, amounts of H3O+ and OH-.
In pure water, the molar concentrations of H3O+ and OH- are each 1.0 x 10^-7 M.
The ion product constant for water:
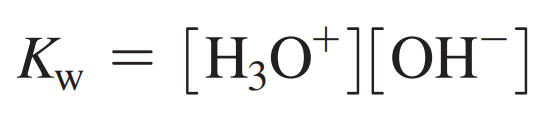
In acidic solutions, the [H3O+] is greater than the [OH-].
In basic solutions, the [OH-] is greater than the [H3O+].
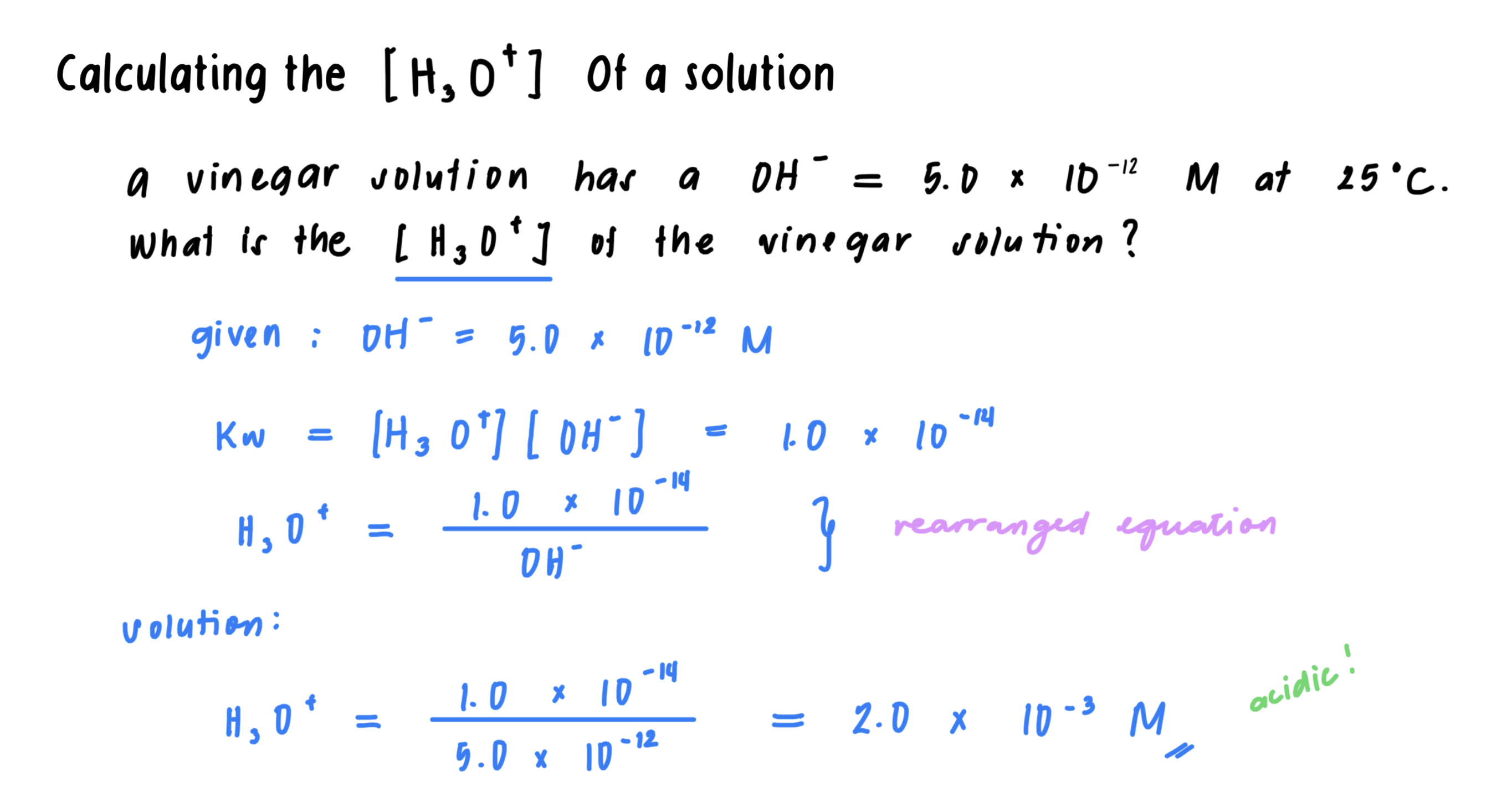
10.5: The pH Scale
pH Scale: A range of numbers typically from 0 to 14, which represents the [H3O+] of the solution.
A neutral solution has a pH of 7.0.
In acidic solutions, the pH is below 7.0.
In basic solutions, the pH is above 7.0.
Mathematically, pH is the negative logarithm of the hydronium ion concentration, pH = –log [H3O+].
10.6: Reactions of Acid and Bases
Salt: An ionic compound that does not have H+ as the cation or OH- as the anion.
Acids react with certain metals to produce hydrogen gas and salt.
Metals that react with acids include potassium, sodium, calcium, magnesium, aluminum, zinc, iron, and tin.
When an acid is added to a carbonate or bicarbonate, the products are carbon dioxide gas, water, and an ionic compound.
Neutralization: A reaction between an acid and a base to produce water and salt.
Titration: A laboratory procedure in which we neutralize an acid sample with a known amount of base.
In the titration, we neutralize the acid by adding a volume of base that contains a matching number of moles of OH-.
We know that neutralization has taken place when the phenolphthalein in the solution changes from colorless to pink. This is called the neutralization endpoint.
From the volume added and molarity of the NaOH solution, we can calculate the number of moles of NaOH, the moles of acid, and then the concentration of the acid.

Antacids: These are substances used to neutralize excess stomach acid.
10.7: Buffers
Buffer Solution: It maintains pH by neutralizing small amounts of added acid or base.
A buffer contains either a weak acid and its salt or a weak base and its salt.
When an acid or base is added to a buffer solution, there is little change in pH.
Acidosis: A condition that occurs when there’s an increase in the CO2 level that leads to a low blood pH concentration.
Alkalosis: A condition that occurs when there’s a decrease in the CO2 level that leads to a high blood pH concentration.
 Knowt
Knowt
