
3.1-3.7: Atoms, Elements and Compounds
Atoms are the smallest particles of matter, that we cannot break down further by chemical means.
Elements Contain only one kind of atom, example sodium.
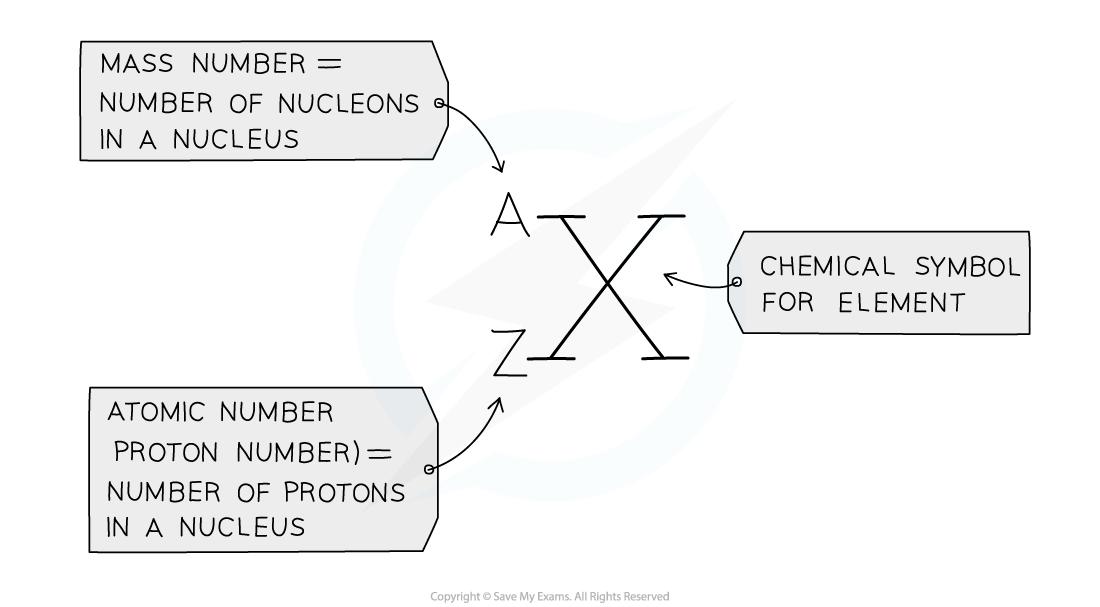
Defining Proton Number
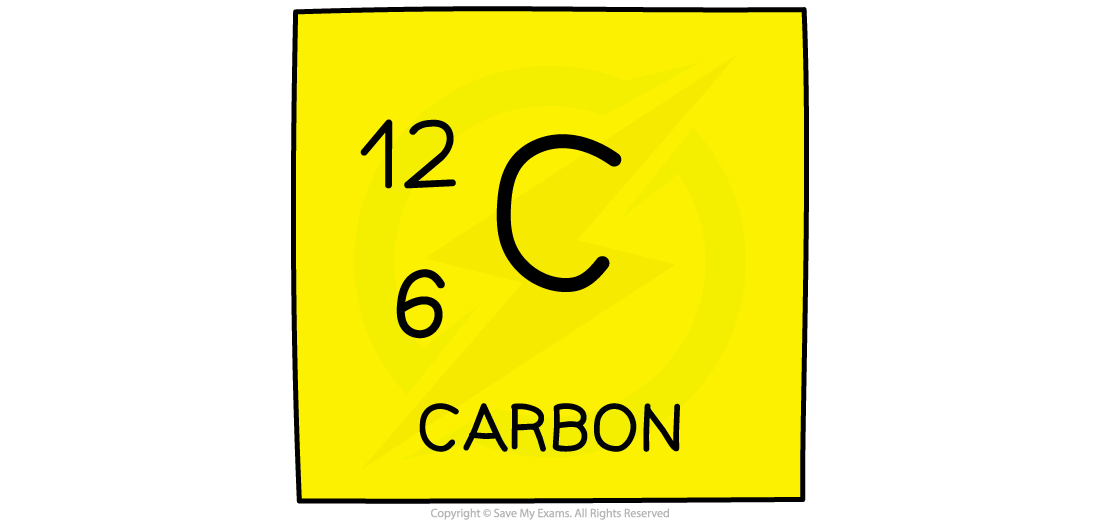
The atomic number (or proton number) is the number of protons in the nucleus of an atom
The symbol for atomic number is Z
It is also the number of electrons present in a neutral atom and determines the position of the element on the Periodic Table.
Defining Nucleon Number
Nucleon number (or mass number) is the total number of protons and neutrons in the nucleus of an atom
The symbol for nucleon number is A
how to find neutrons number: The nucleon number minus the proton number gives you the number of neutrons of an atom
protons + neutrons are called nucleons.
The atomic number and mass number for every element is on the Periodic Table
Electrons
The symbol for an electron is e-, but other symbols are used such as x and ⦁ in bonding diagrams to make it easier to see where electrons come from.
These subatomic particles move very fast around the nucleus.
They move in orbital paths called shells
The mass of the electron is negligible, hence the mass of an atom is concentrated in the nucleus where the neutrons and protons are found.
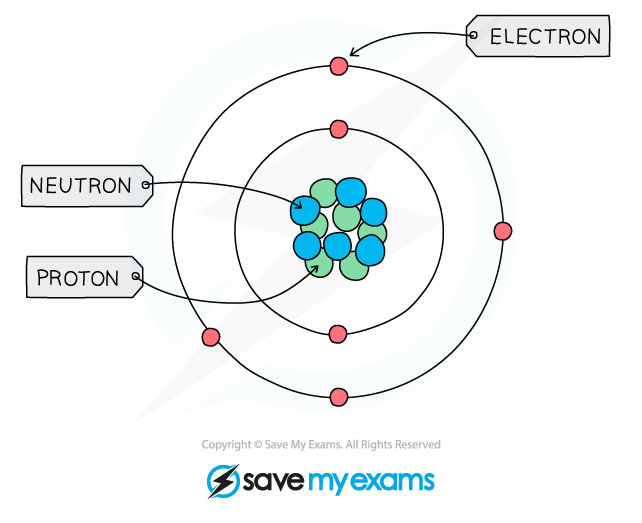
Protons neutrons and elements
Elements are made of tiny particles of matter called atoms
Each atom is made of subatomic particles called protons, neutrons and electrons
Their size is so tiny that we can't really compare their masses in conventional units such as kilograms or grams, so a unit called the relative atomic mass is used
One relative atomic mass unit is equal to 1/12th the mass of a carbon-12 atom.
All other elements are measured relative to the mass of a carbon-12 atom, so relative atomic mass has no units (although sometimes you may come across a unit Da or u which stands for a Dalton and it means the same thing).
Hydrogen for example has a relative atomic mass of 1, meaning that 12 atoms of hydrogen would have exactly the same mass as 1 atom of carbon
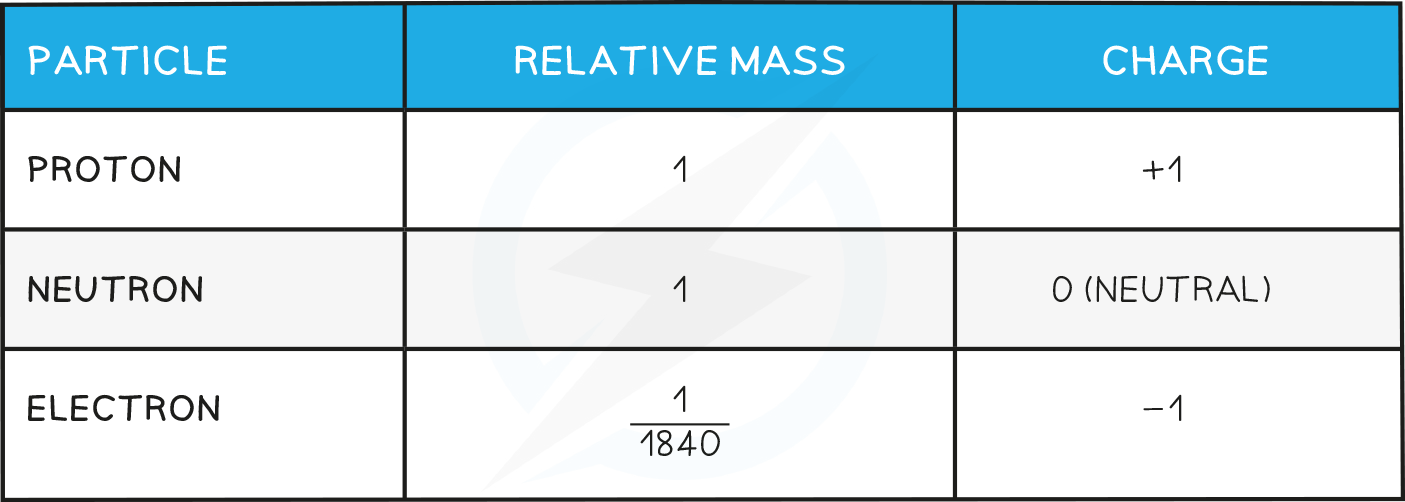
The relative mass and charge of the subatomic particles are shown below:
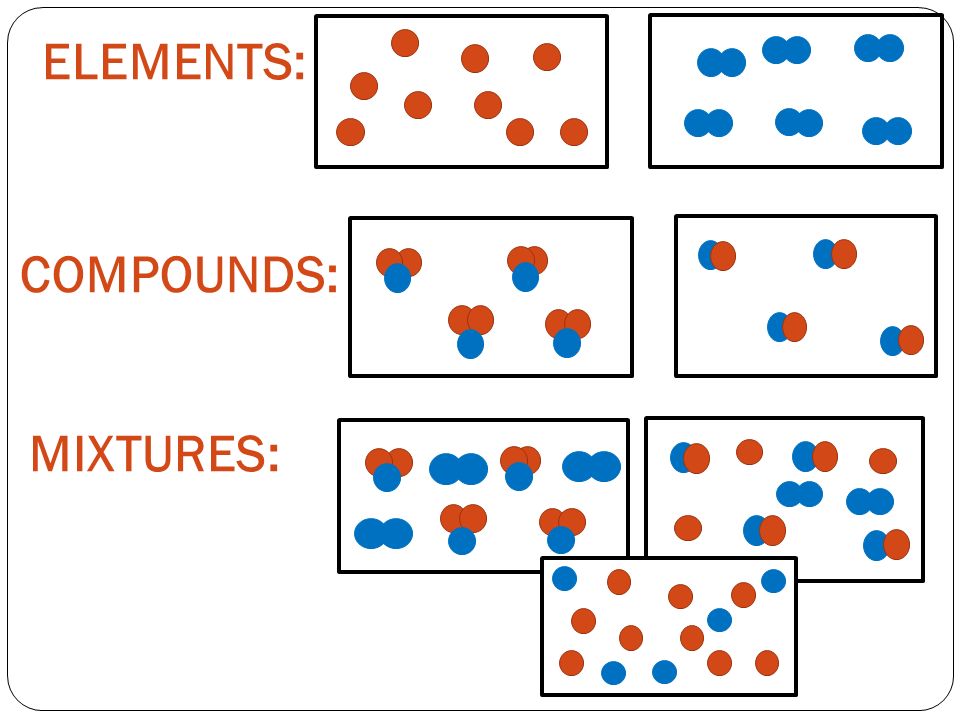
Types of Substances and there Properties
Compound
A pure substance made up of two or more elements chemically combined.
There is an unlimited number of compounds
Compounds cannot be separated into their elements by physical means.
example: Copper sulfate and calcium carbonate Mixture
A combination of two or more substances (elements and/or compounds) that are not chemically combined
Mixtures can be separated by physical methods such as filtration or evaporation
Eg: sand and water, oil and water, sulphur powder and iron filings
Electron Shells
We can represent the structure of the atom in two ways: using diagrams called electron shell diagrams or by writing out a special notation called the electronicstructure
Electron shell diagrams
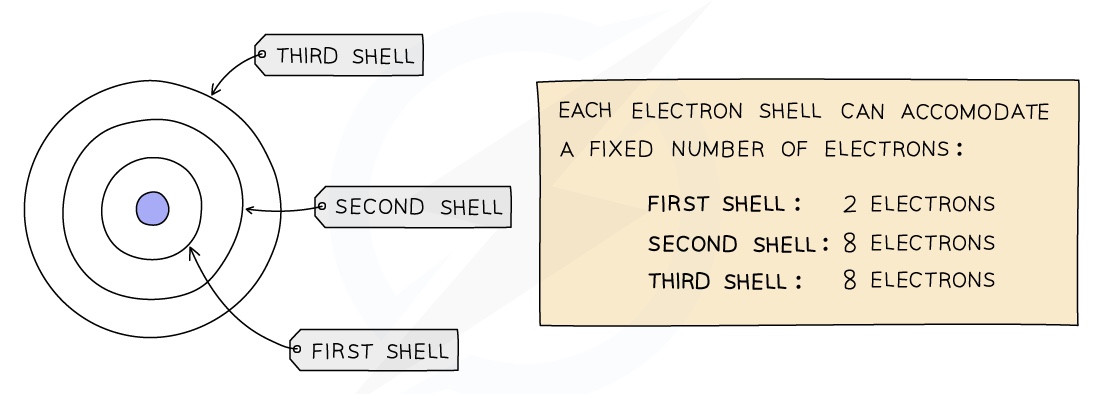
Electrons orbit the nucleus in shells (or energy levels) and each shell has a different amount of energy associated with it
The further away from the nucleus then the more energy a shell has
Electrons occupy the shell closest to the nucleus which can hold only 2 electrons
When a shell becomes full electrons then fill the next shell
The second shell can hold 8 electrons and the third shell can also hold 8 electrons and the electrons organise themselves in pairs in these shells
The outermost shell of an atom is called the valence shell and an atom is much more stable if it can manage to completely fill this shell with electrons
Elements in the same group have the same number of outer shell electrons
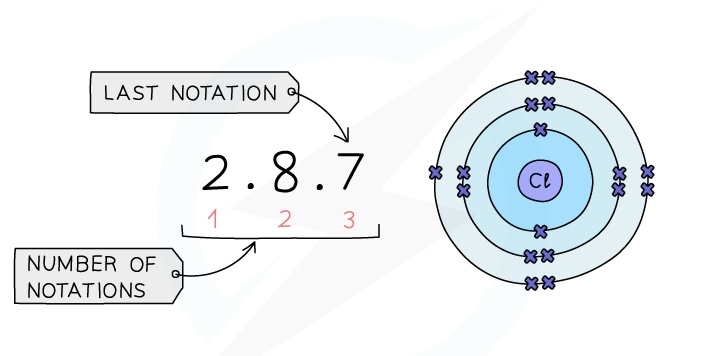
Period: The red numbers at the bottom show the number of notations which is 3, showing that a chlorine atom has 3 shells of electrons
Group: The final notation, which is 7 in the example, shows that a chlorine atom has 7 outer electrons
Noble gases= All of the noble gases are unreactive as they have full outer shells and are thus very stable
located 8/0
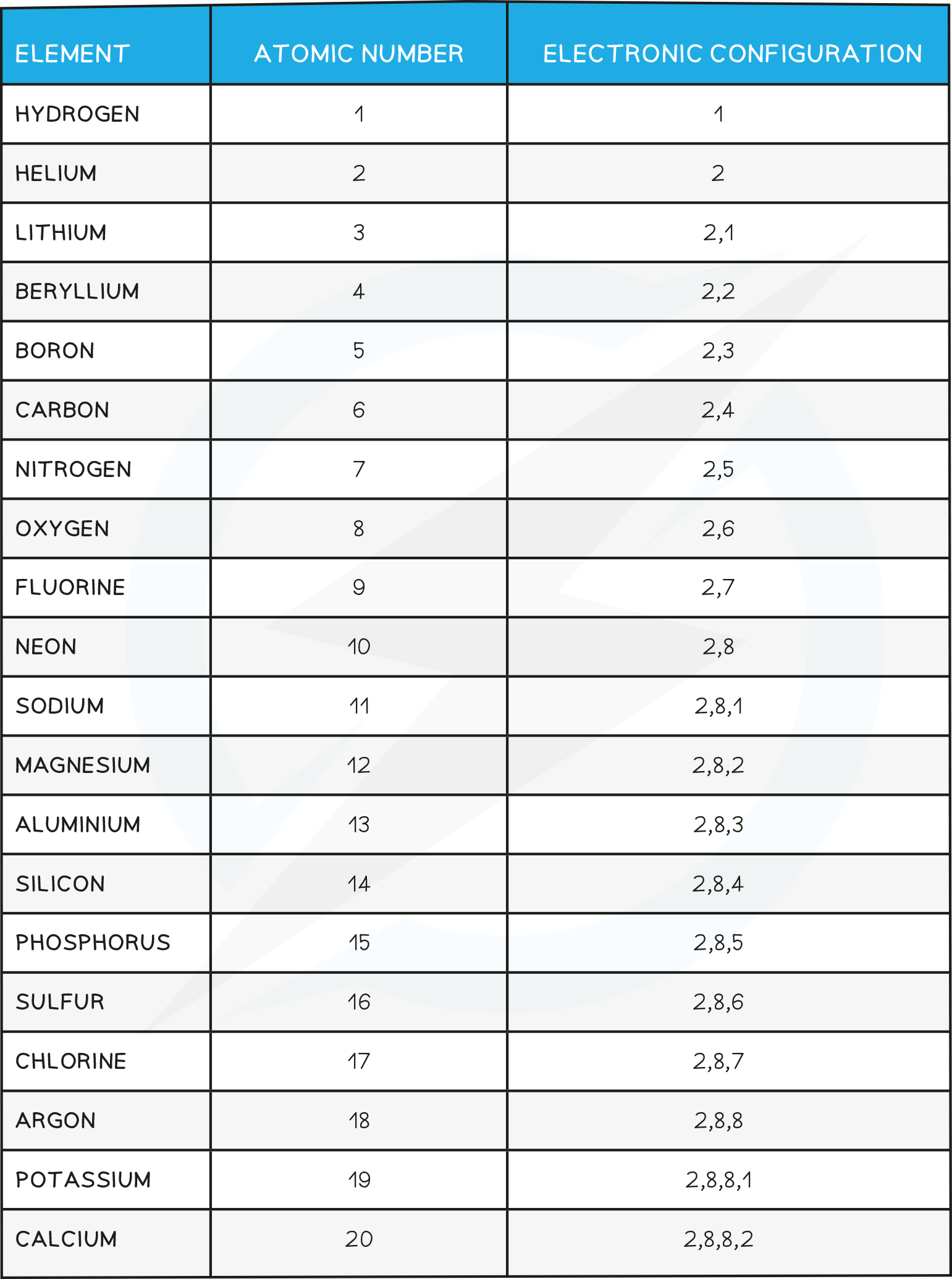
Below is the electronic Configuration of the first 20 elements
The Bases of the Periodic table
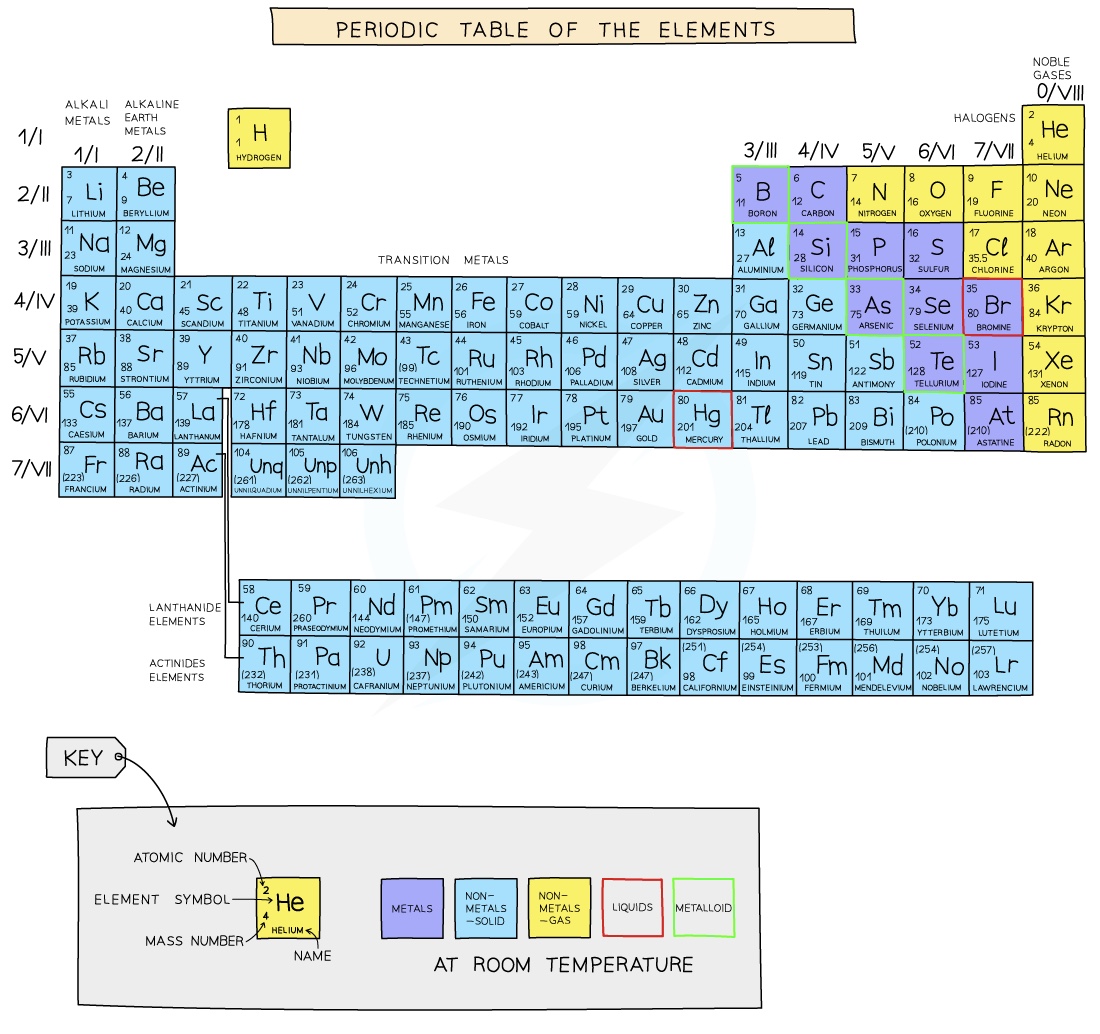
Elements are arranged on the Periodic Table in order of increasing atomic number where each element has one proton more than the element preceding it
Hydrogen has 1 proton, helium has 2 protons, lithium has 3, etc.
The table is arranged in vertical columns called Groupsnumbered I - VIII and in rows called Periods
Elements in the same group have the same amount of electrons in their outer shell, which gives them similar chemicals properties
Ions and ionic bonds
ions
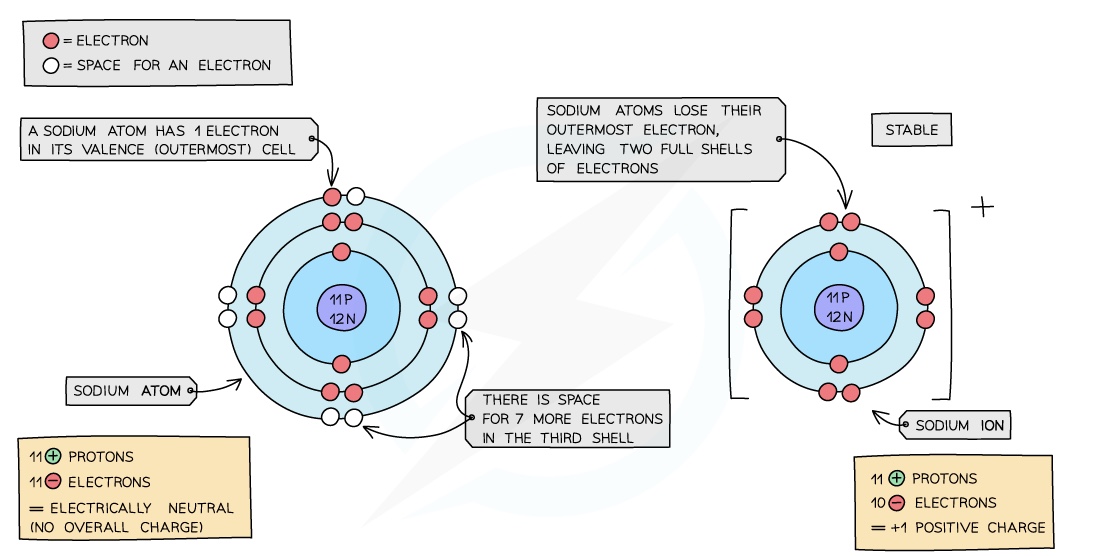
An ion is an electrically charged atom or group of atoms formed by the loss or gain of electrons
An atom will lose or gain electrons to become more stable
The loss or gain of electrons takes place to gain a full outershell of electrons which is a more stable arrangement of electrons
Ionisation of metals and non-metals
Metals: all metals can lose electrons to other atoms to become positively charged ions
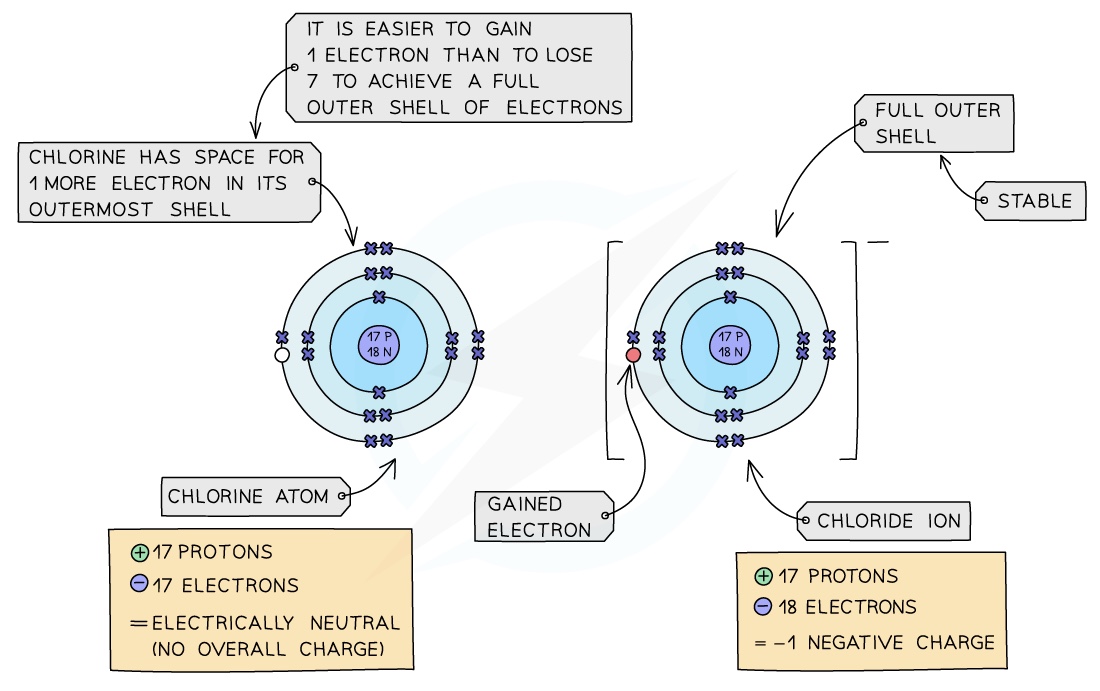
Non-metals: all non-metals can gain electrons from other atoms to become negatively charged ions
Electrostatic attraction
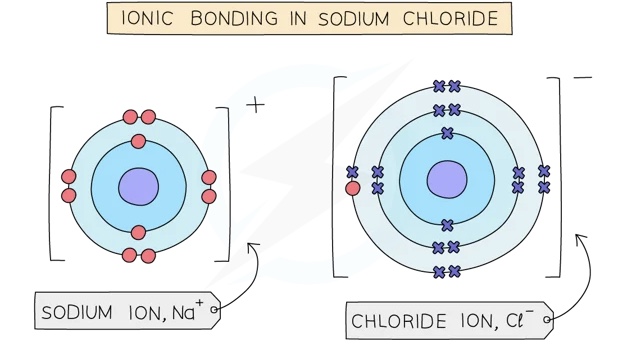
Ionic compounds are formed when metal atoms react with non-metal atoms
Metal atoms lose their outer electrons which the non-metal atoms gain to form positive and negative ions
The positive and negative ions are held together by strong electrostatic forces of attraction between oppositecharges
This force of attraction is known as an ionic bond and they hold ionic compounds together
This happens because formation of ionic bonds, Group 1 to VII
Sodium is a Group I metal so will lose one outer electron to another atom to gain a full outer shell of electrons
A positive sodium ion with the charge 1+ is formed
Chlorine is a Group VII non-metal so will need to gain an electron to have a full outer shell of electrons
One electron will be transferred from the outer shell of the sodium atom to the outer shell of the chlorine atom
A chlorine atom will gain an electron to form a negatively charged chloride ion with a charge of 1-
The oppositely charged ions are held together by strong electrostatic forces of attraction
The ionic compound has no overall charge
Formula of ionic compound: NaCl
Ionic bonds happen between metals and non metals
Lattice structures: In lattice structures, the atoms are arranged in an orderedand repeating fashion
The lattices formed by ionic compounds consist of a regular arrangement of alternating positive and negative ions
Isotopes
Isotopes are atoms of the same element that contain the same number of protonsand electrons but a different number of neutrons.
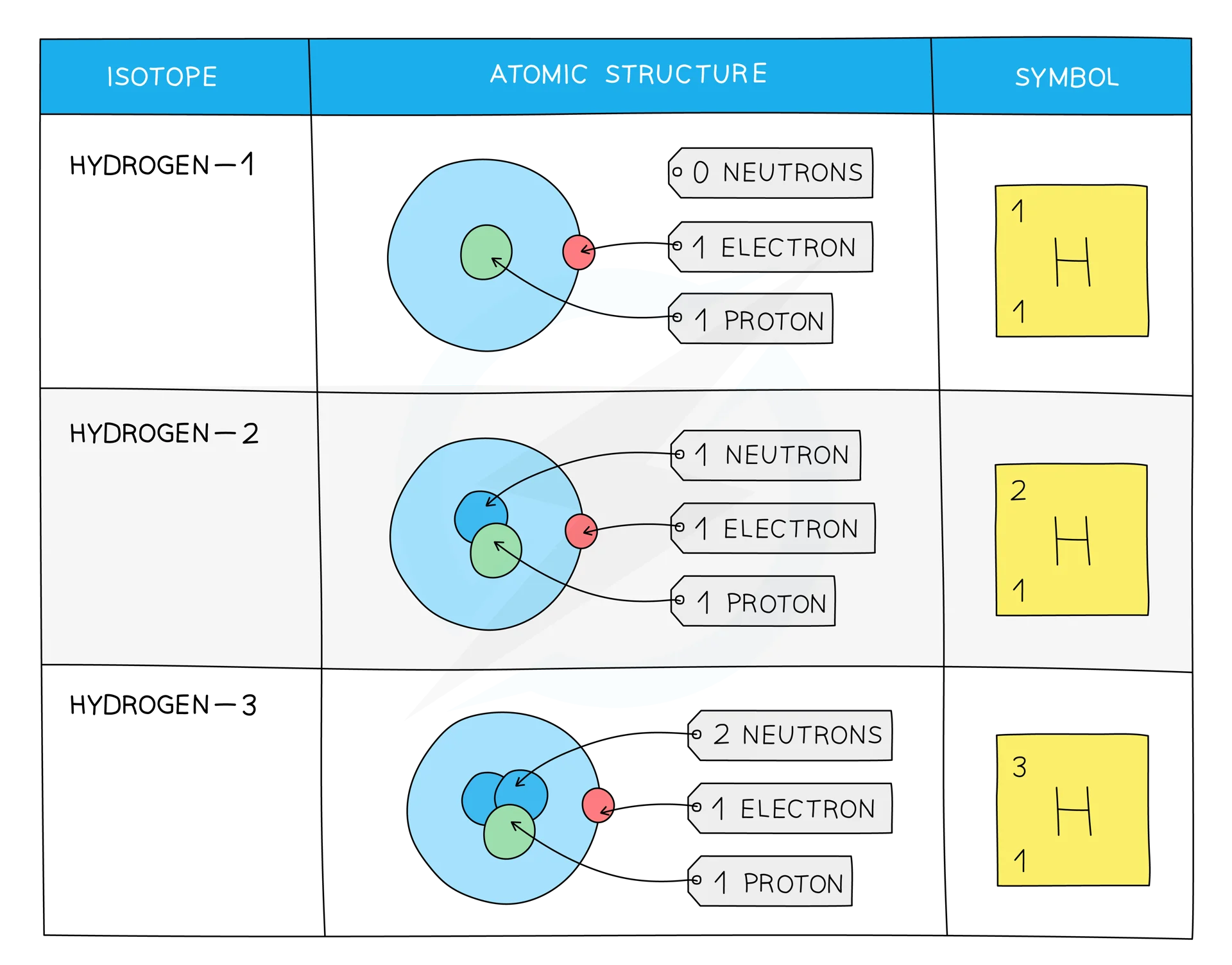
There are two types of Isotopes:
Radioactive
Non Radioactive
Radioactive isotopes
Radioactive isotopes (radioisotopes) are unstable due to the imbalance of neutrons and protons, which causes the nucleus to decay over time through nuclear fission and emit radiation
Examples of radioisotopes include tritium and carbon-14
Radioactive have a lot of use in the medical field
Example= Cancer treatments, Medical tracers, and Medical instruments and materials are routinely sterilized by exposure to radiation, which kills any bacteria present
the industrial uses=
Example= Radioactive tracers are deployed in industry to detect leaks in gas or oil pipes, Geiger Counter, and Radiocarbon dating.
Non Radioactive Isotopes
Non-radioactive isotopes are stable atoms which really only
isotopes share properties, why? how?
Isotopes of the same element display the same chemical characteristics
This is because they have the same number of electrons in their outer shells
The difference between isotopes is the neutrons which are neutral particles within the nucleus and add mass only
The difference in mass affects the physical properties, such as density, boiling point and melting point
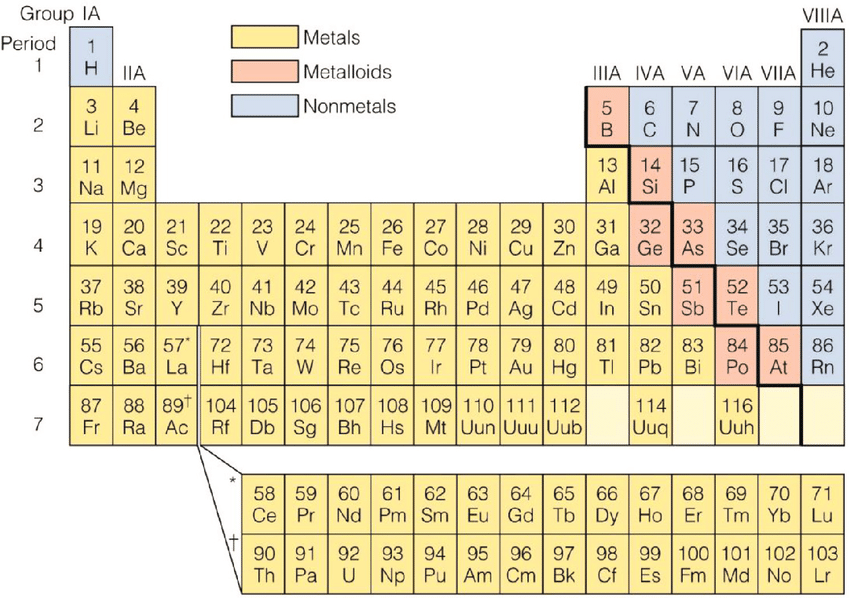
Metals and Non Metals
There are over 100 elements in the Periodic Table
some of them can be classified as metals and nonmetals
Most of the elements are metals and a small number of elements display properties of both types
These elements are called metalloids
Properties of metals
Conduct heat and electricity
Are malleable (can be hammered and made into different shapes) and ductile (can be drawn into wires)
Tend to be lustrous (shiny)
Have high density and usually have high melting points
Form positive ions through electron loss
Form basic oxides
Properties of nonmetal elements
Do not conduct heat and electricity
Are brittle when solid and easily break up
Tend to be dull and nonreflective
Have low density and low melting points (many are gases at room temperature)
Form negative ions through electron gain (except for hydrogen)
Form acidic oxides
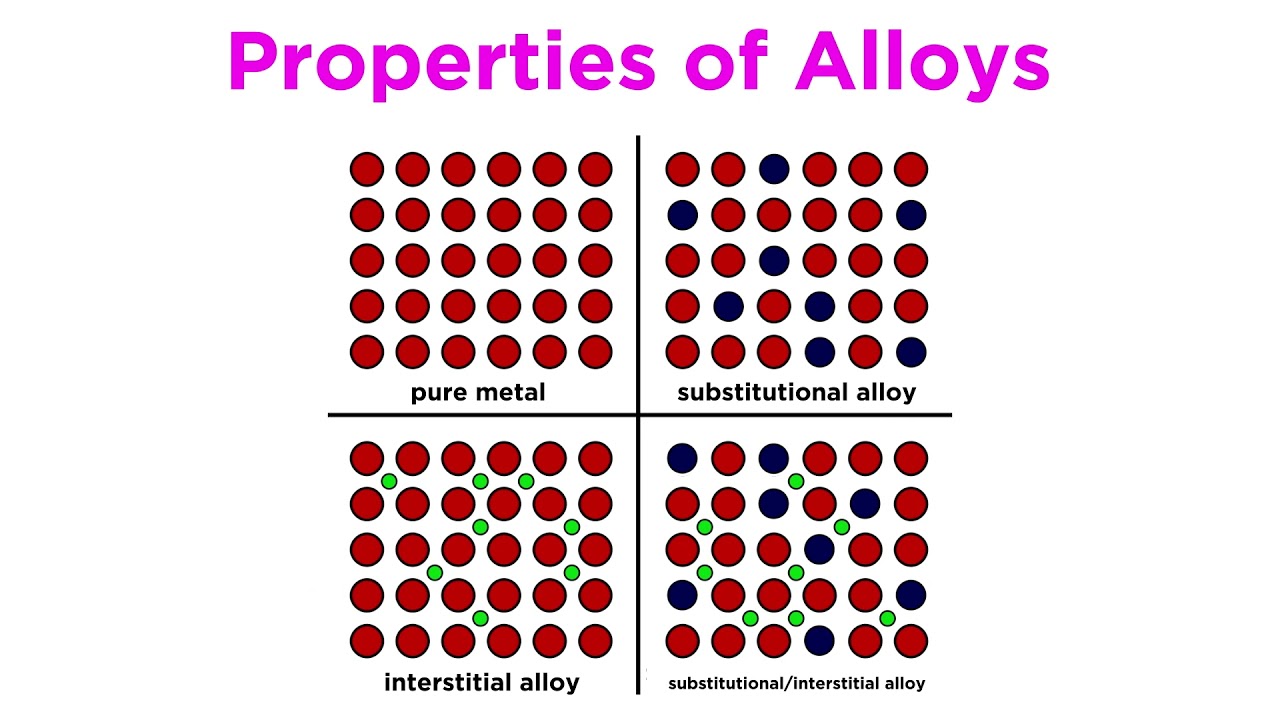
Alloys
Alloys are mixtures of metals, where the metals are mixed together but are not chemically combined
They can also be made from metals mixed with nonmetals such as carbon
Alloys often have properties that can be very different to the metals they contain, for example they can have greater strength, hardness or resistance to corrosion or extreme temperatures
Alloys contain atoms of different sizes, which distorts the regular arrangements of atoms
This makes it more difficult for the layers to slide over each other, so they are usually much harder than the pure metal
Metals have high melting and boiling points
There are many strong metallic bonds in giant metallic structures between the positive metal ion and delocalised electrons
A lot of heat energy is needed to break these bonds
Metals conduct electricity
There are free electrons available to move through the structure and carry charge
Electrons entering one end of the metal cause a delocalised electron to displace itself from the other end
Hence electrons can flow so electricity is conducted
Metals are malleable and ductile
Layers of positive ions can slide over one another and take up different positions
Metallic bonding is not disrupted as the outer electrons do not belong to any particular metal atom so the delocalised electrons will move with them
Metallic bonds are thus not broken and as a result metals are strong but flexible
They can be hammered and bent into different shapes or drawn into wires without breaking
3.1-3.7: Atoms, Elements and Compounds
Atoms are the smallest particles of matter, that we cannot break down further by chemical means.
Elements Contain only one kind of atom, example sodium.
Defining Proton Number
The atomic number (or proton number) is the number of protons in the nucleus of an atom
The symbol for atomic number is Z
It is also the number of electrons present in a neutral atom and determines the position of the element on the Periodic Table.
Defining Nucleon Number
Nucleon number (or mass number) is the total number of protons and neutrons in the nucleus of an atom
The symbol for nucleon number is A
how to find neutrons number: The nucleon number minus the proton number gives you the number of neutrons of an atom
protons + neutrons are called nucleons.
The atomic number and mass number for every element is on the Periodic Table

Electrons
The symbol for an electron is e-, but other symbols are used such as x and ⦁ in bonding diagrams to make it easier to see where electrons come from.
These subatomic particles move very fast around the nucleus.
They move in orbital paths called shells
The mass of the electron is negligible, hence the mass of an atom is concentrated in the nucleus where the neutrons and protons are found.

Protons neutrons and elements
Elements are made of tiny particles of matter called atoms
Each atom is made of subatomic particles called protons, neutrons and electrons
Their size is so tiny that we can't really compare their masses in conventional units such as kilograms or grams, so a unit called the relative atomic mass is used
One relative atomic mass unit is equal to 1/12th the mass of a carbon-12 atom.
All other elements are measured relative to the mass of a carbon-12 atom, so relative atomic mass has no units (although sometimes you may come across a unit Da or u which stands for a Dalton and it means the same thing).
Hydrogen for example has a relative atomic mass of 1, meaning that 12 atoms of hydrogen would have exactly the same mass as 1 atom of carbon
The relative mass and charge of the subatomic particles are shown below:

Types of Substances and there Properties
Compound
A pure substance made up of two or more elements chemically combined.
There is an unlimited number of compounds
Compounds cannot be separated into their elements by physical means.
example: Copper sulfate and calcium carbonate Mixture
A combination of two or more substances (elements and/or compounds) that are not chemically combined
Mixtures can be separated by physical methods such as filtration or evaporation
Eg: sand and water, oil and water, sulphur powder and iron filings

Electron Shells
We can represent the structure of the atom in two ways: using diagrams called electron shell diagrams or by writing out a special notation called the electronicstructure
Electron shell diagrams
Electrons orbit the nucleus in shells (or energy levels) and each shell has a different amount of energy associated with it
The further away from the nucleus then the more energy a shell has
Electrons occupy the shell closest to the nucleus which can hold only 2 electrons
When a shell becomes full electrons then fill the next shell
The second shell can hold 8 electrons and the third shell can also hold 8 electrons and the electrons organise themselves in pairs in these shells
The outermost shell of an atom is called the valence shell and an atom is much more stable if it can manage to completely fill this shell with electrons

Elements in the same group have the same number of outer shell electrons

Period: The red numbers at the bottom show the number of notations which is 3, showing that a chlorine atom has 3 shells of electrons
Group: The final notation, which is 7 in the example, shows that a chlorine atom has 7 outer electrons
Noble gases= All of the noble gases are unreactive as they have full outer shells and are thus very stable
located 8/0
Below is the electronic Configuration of the first 20 elements

The Bases of the Periodic table
Elements are arranged on the Periodic Table in order of increasing atomic number where each element has one proton more than the element preceding it
Hydrogen has 1 proton, helium has 2 protons, lithium has 3, etc.
The table is arranged in vertical columns called Groupsnumbered I - VIII and in rows called Periods
Elements in the same group have the same amount of electrons in their outer shell, which gives them similar chemicals properties


Ions and ionic bonds
ions
An ion is an electrically charged atom or group of atoms formed by the loss or gain of electrons
An atom will lose or gain electrons to become more stable
The loss or gain of electrons takes place to gain a full outershell of electrons which is a more stable arrangement of electrons


Ionisation of metals and non-metals
Metals: all metals can lose electrons to other atoms to become positively charged ions
Non-metals: all non-metals can gain electrons from other atoms to become negatively charged ions
Electrostatic attraction
Ionic compounds are formed when metal atoms react with non-metal atoms
Metal atoms lose their outer electrons which the non-metal atoms gain to form positive and negative ions
The positive and negative ions are held together by strong electrostatic forces of attraction between oppositecharges
This force of attraction is known as an ionic bond and they hold ionic compounds together

This happens because formation of ionic bonds, Group 1 to VII
Sodium is a Group I metal so will lose one outer electron to another atom to gain a full outer shell of electrons
A positive sodium ion with the charge 1+ is formed
Chlorine is a Group VII non-metal so will need to gain an electron to have a full outer shell of electrons
One electron will be transferred from the outer shell of the sodium atom to the outer shell of the chlorine atom
A chlorine atom will gain an electron to form a negatively charged chloride ion with a charge of 1-
The oppositely charged ions are held together by strong electrostatic forces of attraction
The ionic compound has no overall charge
Formula of ionic compound: NaCl
Ionic bonds happen between metals and non metals
Lattice structures: In lattice structures, the atoms are arranged in an orderedand repeating fashion
The lattices formed by ionic compounds consist of a regular arrangement of alternating positive and negative ions
Isotopes
Isotopes are atoms of the same element that contain the same number of protonsand electrons but a different number of neutrons.

There are two types of Isotopes:
Radioactive
Non Radioactive
Radioactive isotopes
Radioactive isotopes (radioisotopes) are unstable due to the imbalance of neutrons and protons, which causes the nucleus to decay over time through nuclear fission and emit radiation
Examples of radioisotopes include tritium and carbon-14
Radioactive have a lot of use in the medical field
Example= Cancer treatments, Medical tracers, and Medical instruments and materials are routinely sterilized by exposure to radiation, which kills any bacteria present
the industrial uses=
Example= Radioactive tracers are deployed in industry to detect leaks in gas or oil pipes, Geiger Counter, and Radiocarbon dating.
Non Radioactive Isotopes
Non-radioactive isotopes are stable atoms which really only
isotopes share properties, why? how?
Isotopes of the same element display the same chemical characteristics
This is because they have the same number of electrons in their outer shells
The difference between isotopes is the neutrons which are neutral particles within the nucleus and add mass only
The difference in mass affects the physical properties, such as density, boiling point and melting point
Metals and Non Metals
There are over 100 elements in the Periodic Table
some of them can be classified as metals and nonmetals
Most of the elements are metals and a small number of elements display properties of both types
These elements are called metalloids

Properties of metals
Conduct heat and electricity
Are malleable (can be hammered and made into different shapes) and ductile (can be drawn into wires)
Tend to be lustrous (shiny)
Have high density and usually have high melting points
Form positive ions through electron loss
Form basic oxides
Properties of nonmetal elements
Do not conduct heat and electricity
Are brittle when solid and easily break up
Tend to be dull and nonreflective
Have low density and low melting points (many are gases at room temperature)
Form negative ions through electron gain (except for hydrogen)
Form acidic oxides
Alloys
Alloys are mixtures of metals, where the metals are mixed together but are not chemically combined
They can also be made from metals mixed with nonmetals such as carbon
Alloys often have properties that can be very different to the metals they contain, for example they can have greater strength, hardness or resistance to corrosion or extreme temperatures
Alloys contain atoms of different sizes, which distorts the regular arrangements of atoms
This makes it more difficult for the layers to slide over each other, so they are usually much harder than the pure metal

Metals have high melting and boiling points
There are many strong metallic bonds in giant metallic structures between the positive metal ion and delocalised electrons
A lot of heat energy is needed to break these bonds
Metals conduct electricity
There are free electrons available to move through the structure and carry charge
Electrons entering one end of the metal cause a delocalised electron to displace itself from the other end
Hence electrons can flow so electricity is conducted
Metals are malleable and ductile
Layers of positive ions can slide over one another and take up different positions
Metallic bonding is not disrupted as the outer electrons do not belong to any particular metal atom so the delocalised electrons will move with them
Metallic bonds are thus not broken and as a result metals are strong but flexible
They can be hammered and bent into different shapes or drawn into wires without breaking
 Knowt
Knowt
