
Chemical Bonding
Bond Types
Atom Type | Bond Type | Characteristic |
|---|---|---|
Metal - nonmetal | Ionic | Electrons are transferred |
Nonmetal - nonmetal | Covalent | Electrons are shared |
Metal - metal | Metallic | Electrons are pooledelectron sea model |
Chemical bonds form to lower the potential energy of an atom. Nucleus-nucleus and electron-electron repulsion as well as nucleus-electron attraction are used to calculate potential energy.
Potential energy is converted to thermal energy when bonds break/form and when electrons and nuclei reorganize.
The ionization energy of a metal is endothermic, meaning removing an electron from a metal requires energy. This is notated as positive heat (+∆H°).
The electron affinity of a nonmetal is exothermic, meaning that gaining an electron releases heat, notated as negative heat (-∆H°).
Lewis Theory
Lewis theory is a simple theory used to predict the properties of molecules. These bonding predictions are made based on valence electrons, which can be shared (covalent) or transferred (ionic) in a chemical bond.
Valence electrons are represented by dots surrounding the elemental symbol. The octet rule tells us that electrons want to have a full valence shell, which will have eight electrons, filling the outermost s and p orbital of the atom. This is the most stable electron configuration for an atom. There are some exceptions such as hydrogen, helium, and lithium which have a duet. This duet comes from the two electrons that can fit in the s orbital. Beryllium can lose two electrons to form a duet but mostly shared its two electrons, having a stable four-electron configuration. Boron can lose three electrons to form a duet, but more commonly shared its three electrons, having six valence electrons. The expanded octet applies to elements with a d orbital, which provides different bonding opportunities for the electrons. 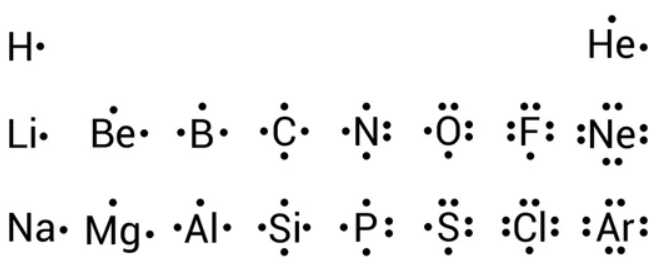
Cations have no valence electrons when stable, which is notated by a positive superscript indicating how many electrons were lost, making that atoms charge more positive.
Anions have a full valence shell which is notated using square brackets around the electrons and a negative superscript showering the electrons gained, making the charge more negative.

Crystalline Lattices
The ionization energy of metals is normally larger than the electron affinity of nonmetals, which should mean that the formation of ionic compounds is endothermic. This is not the case, as it is experimentally proven that the formation of ionic compounds is exothermic. This energy comes from the formation of a crystal lattice, where every anion is surrounded by a cation, and vice versa. Crystal lattices are held together by electrostatic attraction where the attraction is maximized to create the most stable arrangement of ions. Electrostatic attraction is not directional, there is no cation-anion pair, which means there are no ionic molecules in a crystal lattice. The stability of a crystal lattice is measured as lattice energy, which is the energy released when the solid crystal forms from separate ions in the gaseous state. This is always exothermic and depends on the size of the charge and the distance between ions. Large ions have a weaker attraction and therefore a smaller lattice energy. Distance is inversely proportional to energy; as distance increases, energy decreases. Large changes have stronger attraction and therefore larger lattice energy. The charge is directly proportional to the energy. Lattice energy is less exothermic when the ionic radius increases, and more exothermic when the magnitude of ionic charge increases.
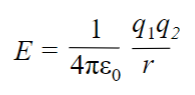
Ionic compounds have high melting and boiling points. They are non-conductive when solid and conductive in solution due to the disassociation of ions. Lewis structure predicts ionic compounds to be brittle.
Born-Haber Cycle
Hess’s law uses the stepwise sum of enthalpy changes to calculate the enthalpy of an unknown molecule.
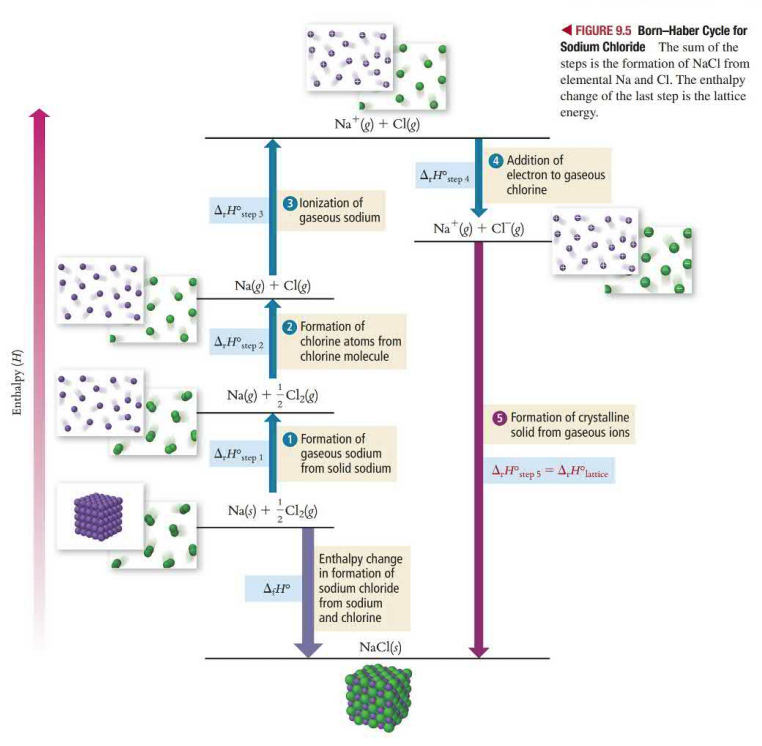
The born-Haber cycle is a series of hypothetical steps that represent the formation of an ionic compound. The steps are chosen all have known enthalpy so the lattice energy can be calculated.
Formation of gaseous metal (+ΔH)
Formation of nonmetal atom (+ΔH)
Ionization of the gaseous metal (+ΔH)
Addition of electron to gaseous nonmetal (-ΔH)
Formation of crystalline solid from gaseous ions (-ΔH)
Covalent Bonds
A shared pair of electrons between two nonmetal atoms is called a bonding pair. Single bonds share two electrons, double bonds share four, and triple bonds share six. A pair of electrons not associated with bonding is called a lone pair or nonbonding electron.
Lone pairs are annotated with two dots on one side of the element’s symbol. A single bond is a line connecting the two atoms, a double bond is two lines and a triple bond is three bonds.
The image above was taken from https://chem.libretexts.org/Courses/Oregon_Institute_of_Technology/OIT%3A_CHE_202_-_General_Chemistry_II/Unit_4%3A_Lewis_Structures/4.2%3A_Lewis_Structures
Covalent molecules have relatively low melting and boiling points in comparison to ionic compounds. Covalent molecules are predicted by Lewis theory to be hard and brittle, some molecules are but others are soft and smooth. Molecules are not conductive when solid or liquid, and molecular acids are conductive when in solution due to being ionized by water.
Polar Covalent Bonds
Polar covalent bonds share electrons unequally, creating partial charges due to different electron densities throughout the molecule. The unequal sharing of electrons creates a dipole. Electronegativity is the ability of an atom to attract an electron, where the large differences in electronegativity creates polar bonds.
Molecules with electronegativity between 0.4 - 2.0 on Linneaus Pauling’s electronegativity scale are said to be polar covalent bonds. Non-polar covalent bonds are between 0.0 - 0.4, pure covalent bonds are exactly 0.0 on the scale, which is seen in diatomics. Ionic bonds have an electronegativity higher than 2.0. 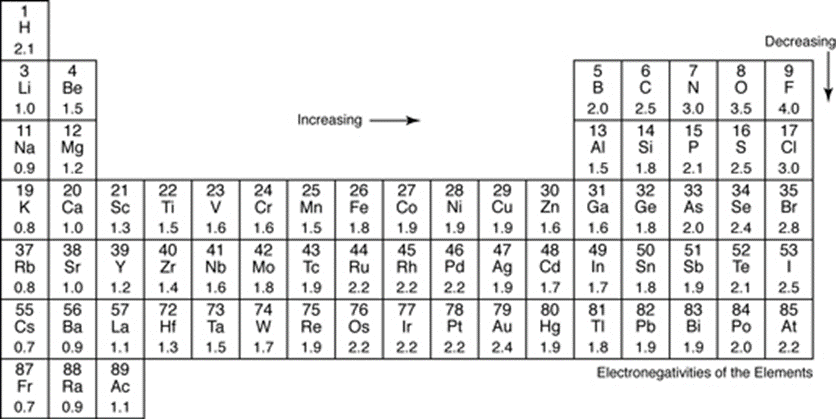
A dipole moment is the measurement of bond polarity that is directly proportional to the size of the distance between them. For a molecule to be polar, the dipole moments of all bonds cannot be zero. The more electronegative an atom is, the more likely electrons will be found around it, creating a negative dipole. If electrons spend less time around an atom, it creates a positive dipole.
Percent Ionic Character
Percent ionic character is the measurement of dipole moment compared to if the electrons were completely transferred. 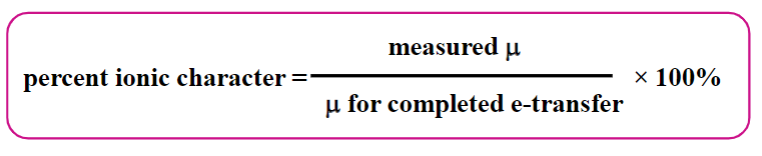
Resonance Structures
Molecules can have resonance, which means there are multiple stable forms they can take. This means there can be various ways to write a Lewis structure and be correct. Resonance structures only vary in the position of electrons, not which atoms are bonded together. Taking into consideration various resonance structures is known as a resonance hybrid. Resonance hybrids are the actual structure of the molecule which takes into account all the valid resonance structures.
Formal Charge
The formal charge of an atom in a molecule is a fake charge given to atoms to help us draw Lewis structures and determine dipoles. The formal charge is the number of valence electrons subtracted by the number of nonbonding electrons and the number of bonds. One bond containing two electrons counts as one in this equation. Formal charge aids in determining if a resonance structure is valid. The lowest possible formal charge yields the most stable resonance structure.
Bond Energy
The amount of energy required to break one mole of a bond is bond energy. This is measured in the gaseous state. The more electrons are shared between two atoms, the higher the bond energy. Short bonds are often strong bonds. Triple bonds are smaller than double bonds which are smaller than single bonds. Average bond energy is used to estimate the enthalpy (∆H) of a system. Bonds breaking release heat (endothermic) and bonds forming need heat (exothermic). The overall enthalpy of a reaction is calculated by the sum of the bonds formed and the bonds broken.
Bond Vibration
Bonds vibrate in various motions which changes their bond length. Energy must be put into the molecule for bonds to vibrate. Infrared spectroscopy is the use of an infrared source to measure the vibration intensity, wave number (inverse of wavelength), and frequency. Bond strength is affected by vibrational energy.
This video shows bond vibrations https://www.youtube.com/watch?v=_vggWQCnC2I
Limitation of Lewis Theory
Lewis theory is able to give qualitative trends (bond length, bond strength, bond angle, molecular geometry) not quantitative trends (exact bond angle). Resonance structures cannot be correctly written using Lewis theory and magnetic behavior is not predicted.
VSEPR Theory
Valence Shell Electron Pair Repulsion Theory is molecular geometry based on electron repulsion on interior atoms. Electrons configured around a central atom will be the most stable when furthest apart from one another. Molecular geometry is based on:
electron groups around the central atom
number of lone pairs/localized electrons
number of bonding groups/delocalized electrons
Molecules are 3D, which can be difficult to illustrate on paper. MolView (https://molview.org/) is a great way to visualize these molecules.

There are five basic arrangements of electrons around a central atom based on a maximum of six bonding electron groups. There can be more than six electrons on large atoms, but it is rare.
Electrons and Molecular Geometry
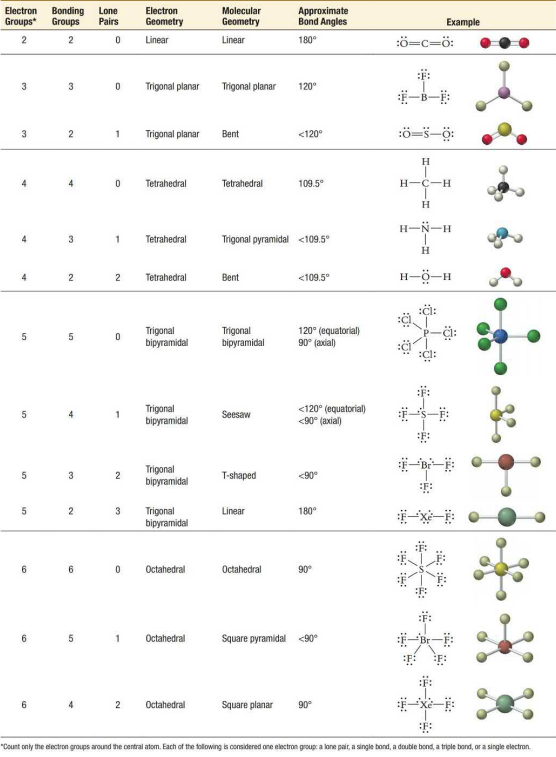
Electron geometry helps visualize molecules, but it is not entirely accurate. Bond type, electronegativity, type of atom, and other factors contribute to actual bond angles, usually by making them smaller than expected. Single bonds occupy less space than double or triple bonds while lone pairs occupy the most space. Delocalized electrons take up less space than localized electrons.
The bond angle varies according to electron repulsion, different electron pairs will have different degrees of repulsion:
Lone pair - lone pair repulsion is the strongest repulsion
Bonding pair - bonding pair is the weakest repulsion
Lone pair - bonding pair is intermediate between the two
A water molecule has bent geometry with a bond angle of 104.5° which is 5° less than the predicted bond angle. This is due to repulsion from oxygens two lone pairs on the hydrogen bonds.
Vector addition of dipole moments can be used to determine polarity. This helps us understand what geometry a molecule has.
Valence Bond Theory
Valence bond theory applies the quantum mechanical model to molecular bonding. Bonds form when atomic orbitals interact, forming a molecular orbital that lowers their potential energy. Molecular orbitals are usually formed from unpaired orbitals that overlap, creating a hybrid orbital with two electrons of opposite spins. Molecular orbital bonds occur between two nuclei and can be outside the axis (pi bonds) or along the axis (sigma bonds). The axis refers to the straight line that can be drawn between two bonded nuclei.
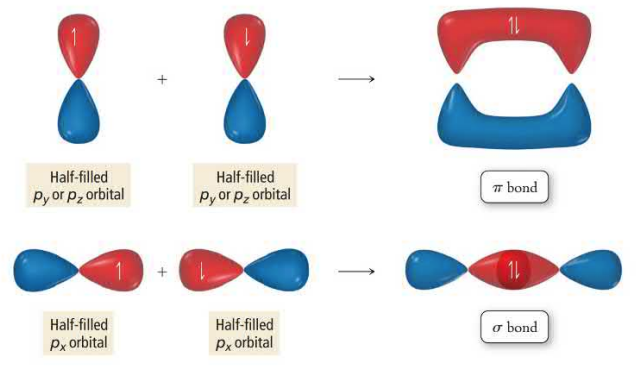
Sigma bonds are interacting orbitals along the axis connecting two nuclei. These bonds are strongest, form first, and are able to rotate. Pi bonds are parallel and perpendicular to the axis. They are weaker than sigma bonds and therefore will be broken first. Pi bonds are unable to rotate which creates resonance structures. Molecules can be cis, meaning the same side, or trans meaning the opposite side.
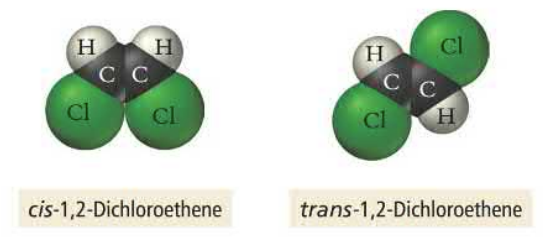
Hybridization is the mathematical mixing of two orbitals, forming hybrid orbitals which more accurately demonstrate the distribution of electrons. Atoms that hybridize their orbitals create degenerate orbitals that maximized orbital overlap to minimize energy. The number of atomic orbitals added together will always equal the number of formed hybrid orbitals.
sp^3 Hybridization
sp^3 orbitals hybridize one s orbital and three p orbitals, creating single bonds which account for the tetrahedral shape of some molecules. Hybridizing reduces electron repulsion by eliminating spin pairs in the same orbital. sp^3 orbitals have three electron groups.
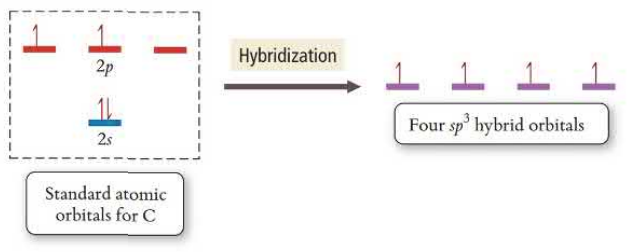
sp^2 Hybridization
sp^2 orbitals hybridize one s orbital and two p orbitals, leaving one p orbital unhybridized. This creates trigonal planar geometry. Double bonds are created by the unhybridized p orbital which is perpendicular to the axis. sp^2 bonds have two electron groups.
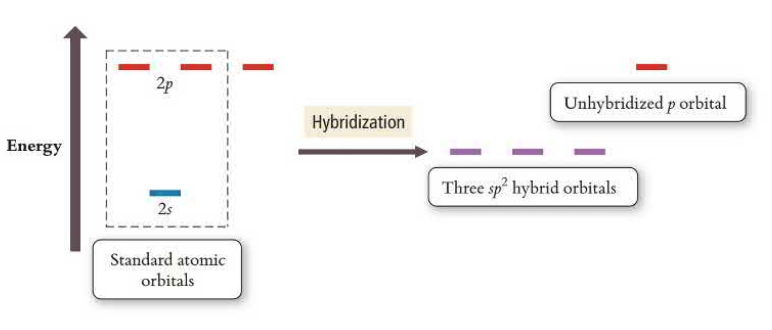
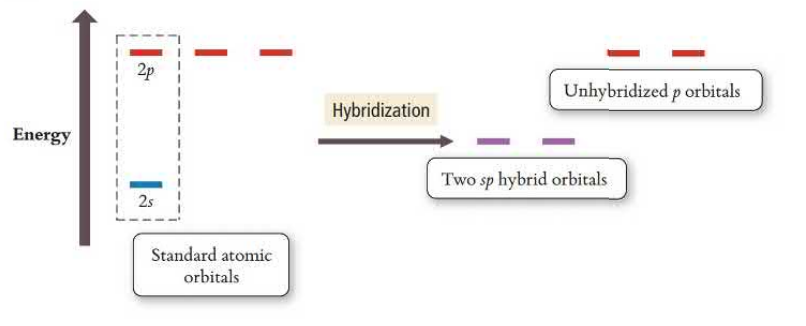
sp Hybridization
sp orbitals hybridize one s orbital and one p orbital, leaving two p orbitals unhybridized. This creates linear geometry and triple bonds that have one sp bond and two unhybridized p orbitals creating pi bonds. sp has two electron groups
Limitations of Valence Bond Theory
Valence bond theory predicts quantitative (bond strength and length) and qualitative properties (bonding scheme and bond rigidity) better than Lewis theory, but does not predict magnetic behaviour and presumes electrons are localized to orbitals on the atom.
Molecular Orbital Theory
The molecular orbital theory uses Schrodinger’s wave equation to calculate molecular orbitals. This theory views electrons as belonging to the whole molecules, meaning they are delocalized. To use this theory, you make educated guesses on what the solution will be and modify it until it satisfies. The energy calculated will always be greater than the actual energy of the orbital.
Linear Combination of Atomic Orbitals
Linear combination of atomic orbitals (LCAO) is the weighted sum of the valence atomic orbitals of all the atoms in a molecule, which considers delocalization. Wave combinations of orbitals can be constructive or destructive.
Constructive wave combinations are called bonding molecular orbitals and have lower energy than their original atomic orbitals. These are normal sigma and pi bonds where the electrons are mostly located between the nuclei.
Destructive wave combinations are called antibonding molecular orbitals and have higher energy than their original atomic orbitals. These have abnormal sigma and pi bonds where there are nodes between the nuclei and the electron density is outside the axis.
The lower energy from the bonding orbital is canceled by the high energy of the anti-bonding orbital
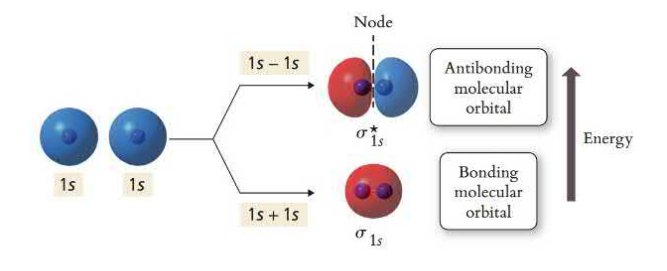
Bond order is the difference in the number of electrons in bonding and antibonding orbitals where only the valence electrons are considered. Higher bond order signifies shorter and stronger bonds. A bond order of less than or equal to zero is unstable in comparison to its atomic form and a bond will not be formed between the two atoms. Unpaired electrons in a molecular orbital diagram show that the molecule is paramagnetic (attracted to a magnetic field). If a molecule’s electrons are all pairs, the substance is diamagnetic (repelled by magnetic fields).

If the bond order is greater than zero, a molecular orbital will form. If the bond order is 0, the energy of the atoms is not lowered by forming bonds therefore molecular orbitals will not form. A zero bond order means there is no net bonding interaction.
The lowest unoccupied molecular orbital (LUMO) is the antibonding orbital and the highest occupied molecular orbital (HOMO) is the bonding orbital. LUMO has higher energy than HOMO and due to its instability.
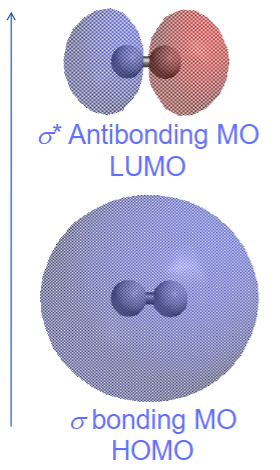
Atomic orbitals of equal energy will contribute to the molecular orbitals equally. Atomic orbitals with different energies will contribute more to the molecular orbitals closest to their energy level. Low energy atomic orbitals contribute to lower energy/bonding molecular orbitals. High energy atomic orbitals will contribute to high energy/antibonding molecular orbitals more. Non-bonding molecular orbitals remain localized on the atom donating its valence electrons.
Chemical Bonding
Bond Types
Atom Type | Bond Type | Characteristic |
|---|---|---|
Metal - nonmetal | Ionic | Electrons are transferred |
Nonmetal - nonmetal | Covalent | Electrons are shared |
Metal - metal | Metallic | Electrons are pooledelectron sea model |
Chemical bonds form to lower the potential energy of an atom. Nucleus-nucleus and electron-electron repulsion as well as nucleus-electron attraction are used to calculate potential energy.
Potential energy is converted to thermal energy when bonds break/form and when electrons and nuclei reorganize.
The ionization energy of a metal is endothermic, meaning removing an electron from a metal requires energy. This is notated as positive heat (+∆H°).
The electron affinity of a nonmetal is exothermic, meaning that gaining an electron releases heat, notated as negative heat (-∆H°).
Lewis Theory
Lewis theory is a simple theory used to predict the properties of molecules. These bonding predictions are made based on valence electrons, which can be shared (covalent) or transferred (ionic) in a chemical bond.
Valence electrons are represented by dots surrounding the elemental symbol. The octet rule tells us that electrons want to have a full valence shell, which will have eight electrons, filling the outermost s and p orbital of the atom. This is the most stable electron configuration for an atom. There are some exceptions such as hydrogen, helium, and lithium which have a duet. This duet comes from the two electrons that can fit in the s orbital. Beryllium can lose two electrons to form a duet but mostly shared its two electrons, having a stable four-electron configuration. Boron can lose three electrons to form a duet, but more commonly shared its three electrons, having six valence electrons. The expanded octet applies to elements with a d orbital, which provides different bonding opportunities for the electrons. 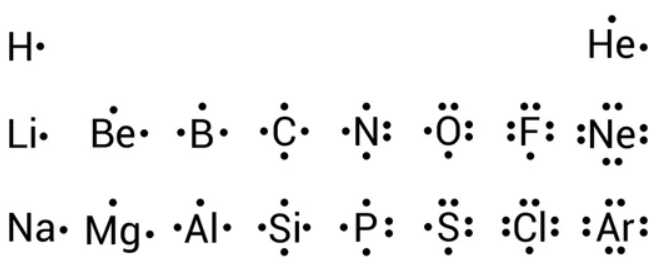
Cations have no valence electrons when stable, which is notated by a positive superscript indicating how many electrons were lost, making that atoms charge more positive.
Anions have a full valence shell which is notated using square brackets around the electrons and a negative superscript showering the electrons gained, making the charge more negative.

Crystalline Lattices
The ionization energy of metals is normally larger than the electron affinity of nonmetals, which should mean that the formation of ionic compounds is endothermic. This is not the case, as it is experimentally proven that the formation of ionic compounds is exothermic. This energy comes from the formation of a crystal lattice, where every anion is surrounded by a cation, and vice versa. Crystal lattices are held together by electrostatic attraction where the attraction is maximized to create the most stable arrangement of ions. Electrostatic attraction is not directional, there is no cation-anion pair, which means there are no ionic molecules in a crystal lattice. The stability of a crystal lattice is measured as lattice energy, which is the energy released when the solid crystal forms from separate ions in the gaseous state. This is always exothermic and depends on the size of the charge and the distance between ions. Large ions have a weaker attraction and therefore a smaller lattice energy. Distance is inversely proportional to energy; as distance increases, energy decreases. Large changes have stronger attraction and therefore larger lattice energy. The charge is directly proportional to the energy. Lattice energy is less exothermic when the ionic radius increases, and more exothermic when the magnitude of ionic charge increases.
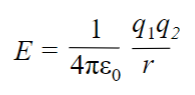
Ionic compounds have high melting and boiling points. They are non-conductive when solid and conductive in solution due to the disassociation of ions. Lewis structure predicts ionic compounds to be brittle.
Born-Haber Cycle
Hess’s law uses the stepwise sum of enthalpy changes to calculate the enthalpy of an unknown molecule.
The born-Haber cycle is a series of hypothetical steps that represent the formation of an ionic compound. The steps are chosen all have known enthalpy so the lattice energy can be calculated.
Formation of gaseous metal (+ΔH)
Formation of nonmetal atom (+ΔH)
Ionization of the gaseous metal (+ΔH)
Addition of electron to gaseous nonmetal (-ΔH)
Formation of crystalline solid from gaseous ions (-ΔH)

Covalent Bonds
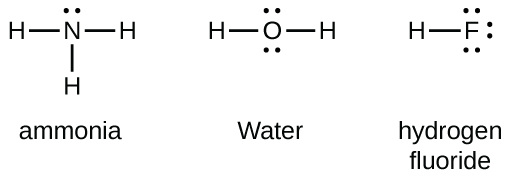
A shared pair of electrons between two nonmetal atoms is called a bonding pair. Single bonds share two electrons, double bonds share four, and triple bonds share six. A pair of electrons not associated with bonding is called a lone pair or nonbonding electron.
Lone pairs are annotated with two dots on one side of the element’s symbol. A single bond is a line connecting the two atoms, a double bond is two lines and a triple bond is three bonds.

The image above was taken from https://chem.libretexts.org/Courses/Oregon_Institute_of_Technology/OIT%3A_CHE_202_-_General_Chemistry_II/Unit_4%3A_Lewis_Structures/4.2%3A_Lewis_Structures
Covalent molecules have relatively low melting and boiling points in comparison to ionic compounds. Covalent molecules are predicted by Lewis theory to be hard and brittle, some molecules are but others are soft and smooth. Molecules are not conductive when solid or liquid, and molecular acids are conductive when in solution due to being ionized by water.
Polar Covalent Bonds
Polar covalent bonds share electrons unequally, creating partial charges due to different electron densities throughout the molecule. The unequal sharing of electrons creates a dipole. Electronegativity is the ability of an atom to attract an electron, where the large differences in electronegativity creates polar bonds.
Molecules with electronegativity between 0.4 - 2.0 on Linneaus Pauling’s electronegativity scale are said to be polar covalent bonds. Non-polar covalent bonds are between 0.0 - 0.4, pure covalent bonds are exactly 0.0 on the scale, which is seen in diatomics. Ionic bonds have an electronegativity higher than 2.0. 
A dipole moment is the measurement of bond polarity that is directly proportional to the size of the distance between them. For a molecule to be polar, the dipole moments of all bonds cannot be zero. The more electronegative an atom is, the more likely electrons will be found around it, creating a negative dipole. If electrons spend less time around an atom, it creates a positive dipole.
Percent Ionic Character
Percent ionic character is the measurement of dipole moment compared to if the electrons were completely transferred. 
Resonance Structures
Molecules can have resonance, which means there are multiple stable forms they can take. This means there can be various ways to write a Lewis structure and be correct. Resonance structures only vary in the position of electrons, not which atoms are bonded together. Taking into consideration various resonance structures is known as a resonance hybrid. Resonance hybrids are the actual structure of the molecule which takes into account all the valid resonance structures.
Formal Charge
The formal charge of an atom in a molecule is a fake charge given to atoms to help us draw Lewis structures and determine dipoles. The formal charge is the number of valence electrons subtracted by the number of nonbonding electrons and the number of bonds. One bond containing two electrons counts as one in this equation. Formal charge aids in determining if a resonance structure is valid. The lowest possible formal charge yields the most stable resonance structure.
Bond Energy
The amount of energy required to break one mole of a bond is bond energy. This is measured in the gaseous state. The more electrons are shared between two atoms, the higher the bond energy. Short bonds are often strong bonds. Triple bonds are smaller than double bonds which are smaller than single bonds. Average bond energy is used to estimate the enthalpy (∆H) of a system. Bonds breaking release heat (endothermic) and bonds forming need heat (exothermic). The overall enthalpy of a reaction is calculated by the sum of the bonds formed and the bonds broken.
Bond Vibration
Bonds vibrate in various motions which changes their bond length. Energy must be put into the molecule for bonds to vibrate. Infrared spectroscopy is the use of an infrared source to measure the vibration intensity, wave number (inverse of wavelength), and frequency. Bond strength is affected by vibrational energy.
This video shows bond vibrations https://www.youtube.com/watch?v=_vggWQCnC2I
Limitation of Lewis Theory
Lewis theory is able to give qualitative trends (bond length, bond strength, bond angle, molecular geometry) not quantitative trends (exact bond angle). Resonance structures cannot be correctly written using Lewis theory and magnetic behavior is not predicted.
VSEPR Theory
Valence Shell Electron Pair Repulsion Theory is molecular geometry based on electron repulsion on interior atoms. Electrons configured around a central atom will be the most stable when furthest apart from one another. Molecular geometry is based on:
electron groups around the central atom
number of lone pairs/localized electrons
number of bonding groups/delocalized electrons
Molecules are 3D, which can be difficult to illustrate on paper. MolView (https://molview.org/) is a great way to visualize these molecules.

There are five basic arrangements of electrons around a central atom based on a maximum of six bonding electron groups. There can be more than six electrons on large atoms, but it is rare.
Electrons and Molecular Geometry

Electron geometry helps visualize molecules, but it is not entirely accurate. Bond type, electronegativity, type of atom, and other factors contribute to actual bond angles, usually by making them smaller than expected. Single bonds occupy less space than double or triple bonds while lone pairs occupy the most space. Delocalized electrons take up less space than localized electrons.
The bond angle varies according to electron repulsion, different electron pairs will have different degrees of repulsion:
Lone pair - lone pair repulsion is the strongest repulsion
Bonding pair - bonding pair is the weakest repulsion
Lone pair - bonding pair is intermediate between the two
A water molecule has bent geometry with a bond angle of 104.5° which is 5° less than the predicted bond angle. This is due to repulsion from oxygens two lone pairs on the hydrogen bonds.
Vector addition of dipole moments can be used to determine polarity. This helps us understand what geometry a molecule has.
Valence Bond Theory
Valence bond theory applies the quantum mechanical model to molecular bonding. Bonds form when atomic orbitals interact, forming a molecular orbital that lowers their potential energy. Molecular orbitals are usually formed from unpaired orbitals that overlap, creating a hybrid orbital with two electrons of opposite spins. Molecular orbital bonds occur between two nuclei and can be outside the axis (pi bonds) or along the axis (sigma bonds). The axis refers to the straight line that can be drawn between two bonded nuclei.

Sigma bonds are interacting orbitals along the axis connecting two nuclei. These bonds are strongest, form first, and are able to rotate. Pi bonds are parallel and perpendicular to the axis. They are weaker than sigma bonds and therefore will be broken first. Pi bonds are unable to rotate which creates resonance structures. Molecules can be cis, meaning the same side, or trans meaning the opposite side.

Hybridization is the mathematical mixing of two orbitals, forming hybrid orbitals which more accurately demonstrate the distribution of electrons. Atoms that hybridize their orbitals create degenerate orbitals that maximized orbital overlap to minimize energy. The number of atomic orbitals added together will always equal the number of formed hybrid orbitals.
sp^3 Hybridization
sp^3 orbitals hybridize one s orbital and three p orbitals, creating single bonds which account for the tetrahedral shape of some molecules. Hybridizing reduces electron repulsion by eliminating spin pairs in the same orbital. sp^3 orbitals have three electron groups.

sp^2 Hybridization
sp^2 orbitals hybridize one s orbital and two p orbitals, leaving one p orbital unhybridized. This creates trigonal planar geometry. Double bonds are created by the unhybridized p orbital which is perpendicular to the axis. sp^2 bonds have two electron groups.

sp Hybridization
sp orbitals hybridize one s orbital and one p orbital, leaving two p orbitals unhybridized. This creates linear geometry and triple bonds that have one sp bond and two unhybridized p orbitals creating pi bonds. sp has two electron groups

Limitations of Valence Bond Theory
Valence bond theory predicts quantitative (bond strength and length) and qualitative properties (bonding scheme and bond rigidity) better than Lewis theory, but does not predict magnetic behaviour and presumes electrons are localized to orbitals on the atom.
Molecular Orbital Theory
The molecular orbital theory uses Schrodinger’s wave equation to calculate molecular orbitals. This theory views electrons as belonging to the whole molecules, meaning they are delocalized. To use this theory, you make educated guesses on what the solution will be and modify it until it satisfies. The energy calculated will always be greater than the actual energy of the orbital.
Linear Combination of Atomic Orbitals
Linear combination of atomic orbitals (LCAO) is the weighted sum of the valence atomic orbitals of all the atoms in a molecule, which considers delocalization. Wave combinations of orbitals can be constructive or destructive.
Constructive wave combinations are called bonding molecular orbitals and have lower energy than their original atomic orbitals. These are normal sigma and pi bonds where the electrons are mostly located between the nuclei.
Destructive wave combinations are called antibonding molecular orbitals and have higher energy than their original atomic orbitals. These have abnormal sigma and pi bonds where there are nodes between the nuclei and the electron density is outside the axis.
The lower energy from the bonding orbital is canceled by the high energy of the anti-bonding orbital

Bond order is the difference in the number of electrons in bonding and antibonding orbitals where only the valence electrons are considered. Higher bond order signifies shorter and stronger bonds. A bond order of less than or equal to zero is unstable in comparison to its atomic form and a bond will not be formed between the two atoms. Unpaired electrons in a molecular orbital diagram show that the molecule is paramagnetic (attracted to a magnetic field). If a molecule’s electrons are all pairs, the substance is diamagnetic (repelled by magnetic fields).

If the bond order is greater than zero, a molecular orbital will form. If the bond order is 0, the energy of the atoms is not lowered by forming bonds therefore molecular orbitals will not form. A zero bond order means there is no net bonding interaction.
The lowest unoccupied molecular orbital (LUMO) is the antibonding orbital and the highest occupied molecular orbital (HOMO) is the bonding orbital. LUMO has higher energy than HOMO and due to its instability.

Atomic orbitals of equal energy will contribute to the molecular orbitals equally. Atomic orbitals with different energies will contribute more to the molecular orbitals closest to their energy level. Low energy atomic orbitals contribute to lower energy/bonding molecular orbitals. High energy atomic orbitals will contribute to high energy/antibonding molecular orbitals more. Non-bonding molecular orbitals remain localized on the atom donating its valence electrons.
 Knowt
Knowt
