
ZOOLOGY(Lecture notes for chapter 4)
CHAPTER 4: Cellular Metabolism
Deferring the Second Law
Living systems appear to contradict the second law of thermodynamics, which states that energy in the universe has direction and that it has been, and always will be, increasing in disorder and randomness and ultimately degraded to heat. This process is termed entropy.
Living systems, however, decrease their entropy by increasing the molecular orderliness of their structure.
All cells must obtain energy,
synthesize their own internal structure,
control much of their own activity,
and guard their boundaries.
Cellular metabolism refers to the collective chemical processes that occur within living cells to accomplish these activities.
4.1 ENERGY AND THE LAWS OF THERMODYNAMICS
The concept of energy is fundamental to all life processes.
Energy cannot be seen; it can be identified only by how it affects matter
Potential energy is stored energy, energy that is not doing work but has the capacity to do so
It can be transformed into
kinetic energy, which can do work and is often termed the energy of motion.
The first law of thermodynamics states
that energy cannot be created or destroyed. It can change from one form to another, but the total amount of energy remains the same.
The second law of thermodynamics states
that a closed system moves toward increasing disorder, or entropy, as energy is dissipated from the system.
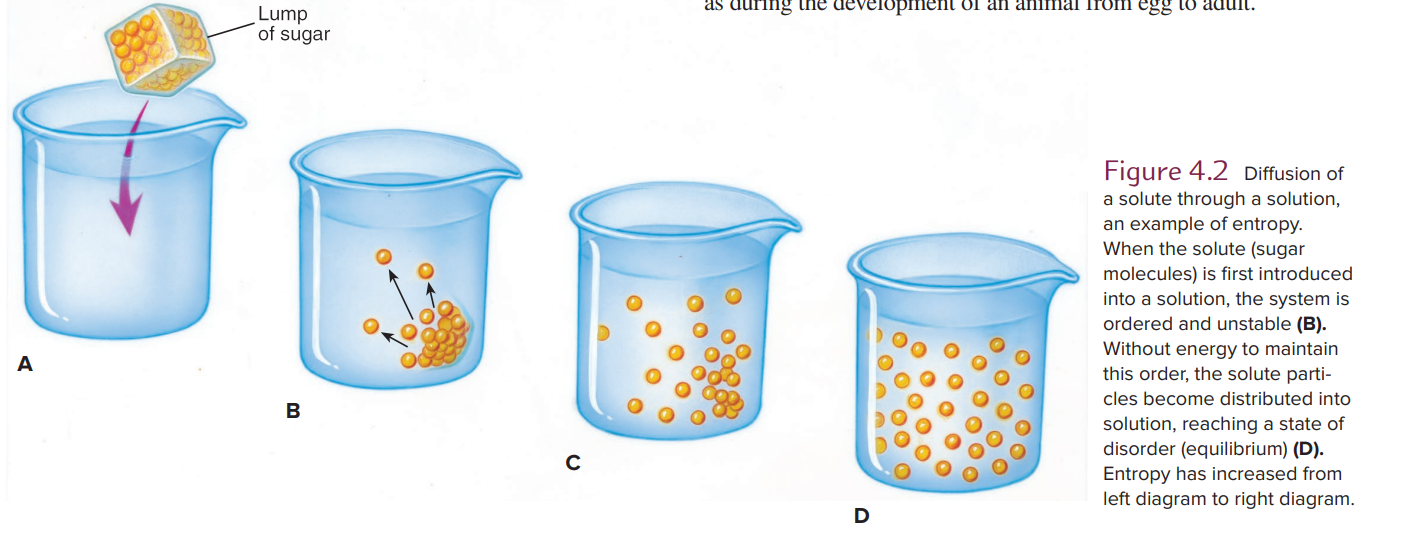
Diffusion and Entropy
-Diffusion of a solute through a solution is an example of entropy
Free Energy
Free energy is simply the energy in a system available for doing work.
In a molecule, free energy equals the energy present in chemical bonds minus the energy that cannot be used.
Exergonic reactions
**-**Release free energy
The majority of reaction in cells are of this type.
-Occurs spontaneously and proceeds downhill.
Endergonic Energy
**-**Reactions requiring the addition of free energy.
-Must be pushed uphill.
-ATP is the molecule used by organisms to power endogenic reactions.
4.2 THE ROLE OF ENZYMES
Enzymes and Activation Energy
For any reaction to occur, even exergonic ones that tend to proceed spontaneously, chemical bonds first must be destabilized.
Activation energy, must be supplied before the bond is stressed enough to break.
Instead, living systems have evolved a different strategy: they employ catalysts.
Catalysts are chemical substances that accelerate reaction rates without affecting the products of the reaction and without being altered or destroyed by the reaction.
Enzymes are catalysts of the living world. They reduce the amount of activation energy required for a reaction.
Note that enzymes do not supply the activation energy. Instead, they lower the activation energy barrier, making a reaction more likely to proceed. Enzymes affect only the reaction rate. They do not in any way alter the free energy change of a reaction.
Nature of Enzymes
Enzymes are complex molecules that vary in size from small, simple molecules with a molecular weight of 10,000 to highly complex molecules with molecular weights up to 1 million. Most enzymes are proteins—highly folded and interlinked chains of amino acids.
Some require participation of small nonprotein groups called cofactors to perform their enzymatic function. In some cases these cofactors are metallic ions.
Another class of cofactors, called coenzymes, is organic. Coenzymes contain groups derived from vitamins, most of which must be supplied in the diet. All B-complex vitamins are coenzymatic compounds.
Another type of molecule, ribonucleic acid (RNA), is now known to possess enzymatic activity. Specifically, ribosomal RNA (rRNA), a major component of ribosomes, provides the activation energy that enables amino acids to assemble into polypeptide chains during the process of translation.
Action of Enzymes
An enzyme functions by associating in a highly specific way with its substrate.
Enzymes have an active site located within a cleft or pocket in a unique molecular configuration.
The binding of enzyme to substrate forms an enzyme-substrate complex (ES complex), in which the substrate is secured by covalent bonds to one or more points in the active site of the enzyme.
The ES complex is not strong and will quickly dissociate, but during this fleeting moment the enzyme provides a unique chemical environment that stresses certain chemical bonds in the substrate so that much less energy is required to complete the reaction

Specificity of Enzymes
Specificity is a consequence of the exact molecular fit required between enzyme and substrate, so that both are specific to each other. Furthermore, an enzyme catalyzes only one reaction. Unlike reactions performed in an organic chemist’s laboratory, no side reactions or by-products result.
Enzyme-Catalyzed Reactions
Enzyme-catalyzed reactions are reversible, which is signified by double arrows between substrate and products, as in this example: Fumaric acid + H2O - Malic acid.
Reactions catalyzed by most enzymes tend to go predominantly in one direction.
The net direction of any chemical reaction depends on the relative energy contents of the substances involved.
In cells both reversible and irreversible reactions are combined in complex ways to make possible both synthesis and degradation.
Hydrolysis and Condensation Reactions
Hydrolysis literally means “breaking with water.” In hydrolysis reactions, a molecule is cleaved by the addition of water at the cleavage site. A hydrogen atom is attached to one subunit and a hydroxyl (—OH) unit is attached to another (see Figure 4.5). This breaks the covalent bond between subunits. Hydrolysis is the opposite of condensation (water-losing) reactions, in which subunits of molecules are linked together by removal of water. Macromolecules are built by condensation reactions.
4.3 ENZYME REGULATION
Mechanisms exist for critically regulating enzymes in both quantity and activity. Genes leading to synthesis of an enzyme may be switched on or off, depending on the presence or absence of a substrate molecule. In this way, the quantity of an enzyme is controlled.
Feedback inhibition- the final end product of a particular metabolic pathway inhibits the first enzyme in the pathway.
4.4 CHEMICAL ENERGY TRANSFER BY ATP
Endergonic reactions are those that do not proceed spontaneously because their products require an input of free energy.
However, an endergonic reaction may be driven by coupling the energy-requiring reaction with an energy-producing reaction.
ATP is one of the most common intermediates in coupled reactions, and because it can drive such energetically unfavorable reactions, it is of central importance in metabolic processes.
ATP molecule consists of adenosine (the purine adenine and the 5-carbon sugar ribose) and a triphosphate group. Most free energy in ATP resides in the triphosphate group, especially in two phosphoanhydride bonds between the phosphate groups called “high-energy bonds”

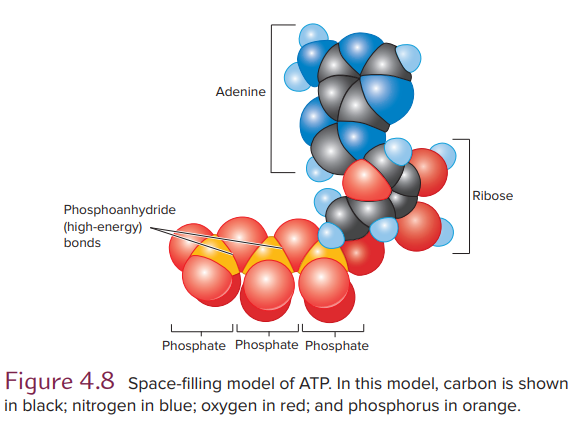
Note that ATP is an energy-coupling agent and not a fuel. It is not a storehouse of energy set aside for some future need. Rather, it is produced by one set of reactions and is almost immediately consumed by another set. ATP is formed as it is needed, primarily by oxidative processes in mitochondria.
Oxygen is not consumed unless ADP and phosphate molecules are available, and these do not become available until ATP is hydrolyzed by some energy-consuming process.
Metabolism is therefore mostly self-regulating.
4.5 CELLULAR RESPIRATION
How Electron Transport Is Used to Trap Chemical Bond Energy
All cells obtain their chemical energy requirements from oxidation-reduction reactions.
This means that in the degradation of fuel molecules, such as glucose, hydrogen atoms (electrons and protons) are passed from electron donors to electron acceptors with a release of energy. A portion of this energy can be trapped and used to form the high-energy bonds of molecules such as ATP.
An oxidation-reduction (“redox”) reaction involves a transfer of electrons from an electron donor (the reducing agent) to an electron acceptor (the oxidizing agent). As soon as the electron donor loses its electrons, it becomes oxidized. As soon as the electron acceptor accepts electrons, it becomes reduced (see Figure 4.10)
Aerobic versus Anaerobic Metabolism
aerobes, those that use molecular oxygen as the final electron acceptor, and anaerobes, those that employ another molecule as the final electron acceptor.
Overview of Respiration
Aerobic metabolism is usually called cellular respiration and is defined as the oxidation of fuel molecules to produce energy with molecular oxygen as the final electron acceptor. Oxidation of fuel molecules describes the removal of electrons from fuel molecules and not the direct combination of molecular oxygen with fuel molecules.
CELLULAR RESPIRATION
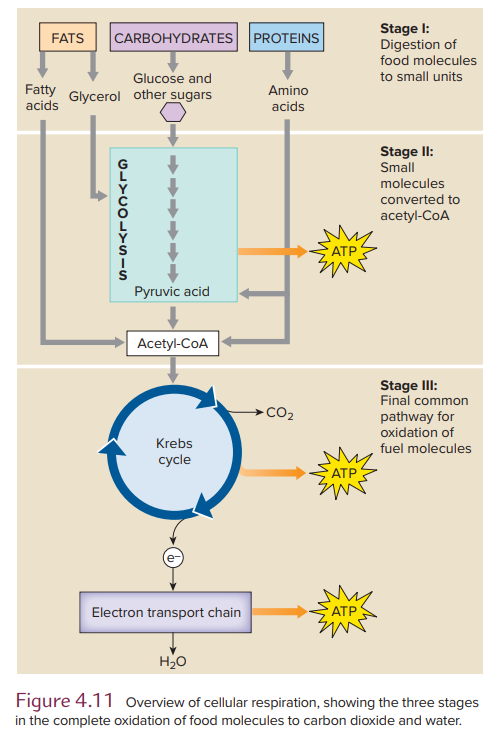
Glycolysis
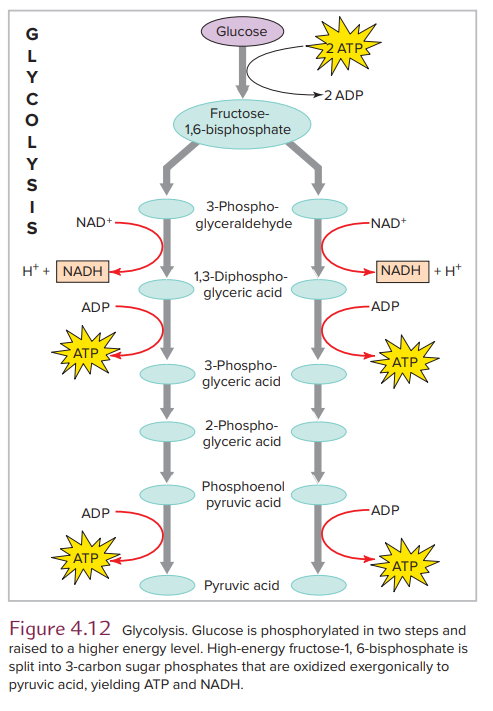
We begin our journey through the stages of respiration with stage II or glycolysis, a nearly universal pathway in living organisms that converts glucose into pyruvic acid. In a series of reactions occurring in the cell cytoplasm, glucose and other 6-carbon monosaccharides are split into 3-carbon molecules of pyruvic acid (see Figure 4.12).
Acetyl-CoA: Strategic Intermediate in Respiration
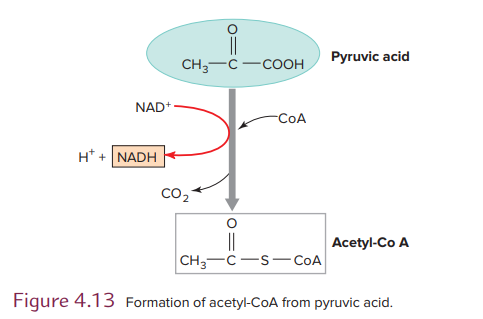
In aerobic metabolis,m the two molecules of pyruvic acid formed during glycolysis enter a mitochondrion. There, each molecule is oxidized, and one of the carbons is released as carbon dioxide (see Figure 4.13). The 2-carbon residue reacts with coenzyme A (CoA) to form acetyl coenzyme A, or acetyl-CoA, and an NADH molecule is also produced.
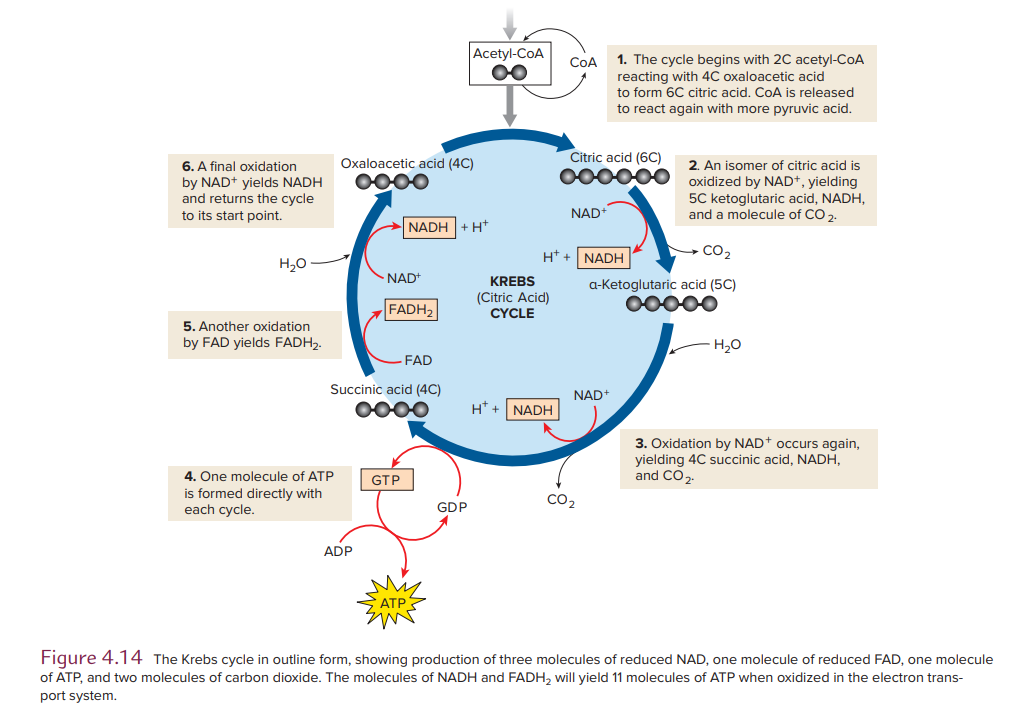
Krebs Cycle: Oxidation of Acetyl-CoA
Oxidation of the 2-carbon acetyl group of acetyl-CoA occurs within the mitochondrial matrix in a cyclic sequence called the Krebs cycle.
Pyruvate or Pyruvic Acid?
Pyruvic acid is the undissociated form of the acid: O CH3—C—COOH Under physiological conditions pyruvic acid typically dissociates into pyruvate O (CH3—C—COO–) and H+. It is correct to use either term in describing this and other organic acids (such as lactic acid or lactate) in metabolism.
ZOOLOGY(Lecture notes for chapter 4)
CHAPTER 4: Cellular Metabolism
Deferring the Second Law
Living systems appear to contradict the second law of thermodynamics, which states that energy in the universe has direction and that it has been, and always will be, increasing in disorder and randomness and ultimately degraded to heat. This process is termed entropy.
Living systems, however, decrease their entropy by increasing the molecular orderliness of their structure.
All cells must obtain energy,
synthesize their own internal structure,
control much of their own activity,
and guard their boundaries.
Cellular metabolism refers to the collective chemical processes that occur within living cells to accomplish these activities.
4.1 ENERGY AND THE LAWS OF THERMODYNAMICS
The concept of energy is fundamental to all life processes.
Energy cannot be seen; it can be identified only by how it affects matter
Potential energy is stored energy, energy that is not doing work but has the capacity to do so
It can be transformed into
kinetic energy, which can do work and is often termed the energy of motion.
The first law of thermodynamics states
that energy cannot be created or destroyed. It can change from one form to another, but the total amount of energy remains the same.
The second law of thermodynamics states
that a closed system moves toward increasing disorder, or entropy, as energy is dissipated from the system.
Diffusion and Entropy
-Diffusion of a solute through a solution is an example of entropy

Free Energy
Free energy is simply the energy in a system available for doing work.
In a molecule, free energy equals the energy present in chemical bonds minus the energy that cannot be used.
Exergonic reactions
**-**Release free energy
The majority of reaction in cells are of this type.
-Occurs spontaneously and proceeds downhill.
Endergonic Energy
**-**Reactions requiring the addition of free energy.
-Must be pushed uphill.
-ATP is the molecule used by organisms to power endogenic reactions.
4.2 THE ROLE OF ENZYMES
Enzymes and Activation Energy
For any reaction to occur, even exergonic ones that tend to proceed spontaneously, chemical bonds first must be destabilized.
Activation energy, must be supplied before the bond is stressed enough to break.
Instead, living systems have evolved a different strategy: they employ catalysts.
Catalysts are chemical substances that accelerate reaction rates without affecting the products of the reaction and without being altered or destroyed by the reaction.
Enzymes are catalysts of the living world. They reduce the amount of activation energy required for a reaction.
Note that enzymes do not supply the activation energy. Instead, they lower the activation energy barrier, making a reaction more likely to proceed. Enzymes affect only the reaction rate. They do not in any way alter the free energy change of a reaction.
Nature of Enzymes
Enzymes are complex molecules that vary in size from small, simple molecules with a molecular weight of 10,000 to highly complex molecules with molecular weights up to 1 million. Most enzymes are proteins—highly folded and interlinked chains of amino acids.
Some require participation of small nonprotein groups called cofactors to perform their enzymatic function. In some cases these cofactors are metallic ions.
Another class of cofactors, called coenzymes, is organic. Coenzymes contain groups derived from vitamins, most of which must be supplied in the diet. All B-complex vitamins are coenzymatic compounds.
Another type of molecule, ribonucleic acid (RNA), is now known to possess enzymatic activity. Specifically, ribosomal RNA (rRNA), a major component of ribosomes, provides the activation energy that enables amino acids to assemble into polypeptide chains during the process of translation.
Action of Enzymes
An enzyme functions by associating in a highly specific way with its substrate.
Enzymes have an active site located within a cleft or pocket in a unique molecular configuration.
The binding of enzyme to substrate forms an enzyme-substrate complex (ES complex), in which the substrate is secured by covalent bonds to one or more points in the active site of the enzyme.
The ES complex is not strong and will quickly dissociate, but during this fleeting moment the enzyme provides a unique chemical environment that stresses certain chemical bonds in the substrate so that much less energy is required to complete the reaction

Specificity of Enzymes
Specificity is a consequence of the exact molecular fit required between enzyme and substrate, so that both are specific to each other. Furthermore, an enzyme catalyzes only one reaction. Unlike reactions performed in an organic chemist’s laboratory, no side reactions or by-products result.
Enzyme-Catalyzed Reactions
Enzyme-catalyzed reactions are reversible, which is signified by double arrows between substrate and products, as in this example: Fumaric acid + H2O - Malic acid.
Reactions catalyzed by most enzymes tend to go predominantly in one direction.
The net direction of any chemical reaction depends on the relative energy contents of the substances involved.
In cells both reversible and irreversible reactions are combined in complex ways to make possible both synthesis and degradation.
Hydrolysis and Condensation Reactions
Hydrolysis literally means “breaking with water.” In hydrolysis reactions, a molecule is cleaved by the addition of water at the cleavage site. A hydrogen atom is attached to one subunit and a hydroxyl (—OH) unit is attached to another (see Figure 4.5). This breaks the covalent bond between subunits. Hydrolysis is the opposite of condensation (water-losing) reactions, in which subunits of molecules are linked together by removal of water. Macromolecules are built by condensation reactions.
4.3 ENZYME REGULATION
Mechanisms exist for critically regulating enzymes in both quantity and activity. Genes leading to synthesis of an enzyme may be switched on or off, depending on the presence or absence of a substrate molecule. In this way, the quantity of an enzyme is controlled.
Feedback inhibition- the final end product of a particular metabolic pathway inhibits the first enzyme in the pathway.
4.4 CHEMICAL ENERGY TRANSFER BY ATP
Endergonic reactions are those that do not proceed spontaneously because their products require an input of free energy.
However, an endergonic reaction may be driven by coupling the energy-requiring reaction with an energy-producing reaction.
ATP is one of the most common intermediates in coupled reactions, and because it can drive such energetically unfavorable reactions, it is of central importance in metabolic processes.
ATP molecule consists of adenosine (the purine adenine and the 5-carbon sugar ribose) and a triphosphate group. Most free energy in ATP resides in the triphosphate group, especially in two phosphoanhydride bonds between the phosphate groups called “high-energy bonds”


Note that ATP is an energy-coupling agent and not a fuel. It is not a storehouse of energy set aside for some future need. Rather, it is produced by one set of reactions and is almost immediately consumed by another set. ATP is formed as it is needed, primarily by oxidative processes in mitochondria.
Oxygen is not consumed unless ADP and phosphate molecules are available, and these do not become available until ATP is hydrolyzed by some energy-consuming process.
Metabolism is therefore mostly self-regulating.
4.5 CELLULAR RESPIRATION
How Electron Transport Is Used to Trap Chemical Bond Energy
All cells obtain their chemical energy requirements from oxidation-reduction reactions.
This means that in the degradation of fuel molecules, such as glucose, hydrogen atoms (electrons and protons) are passed from electron donors to electron acceptors with a release of energy. A portion of this energy can be trapped and used to form the high-energy bonds of molecules such as ATP.
An oxidation-reduction (“redox”) reaction involves a transfer of electrons from an electron donor (the reducing agent) to an electron acceptor (the oxidizing agent). As soon as the electron donor loses its electrons, it becomes oxidized. As soon as the electron acceptor accepts electrons, it becomes reduced (see Figure 4.10)
Aerobic versus Anaerobic Metabolism
aerobes, those that use molecular oxygen as the final electron acceptor, and anaerobes, those that employ another molecule as the final electron acceptor.
Overview of Respiration
Aerobic metabolism is usually called cellular respiration and is defined as the oxidation of fuel molecules to produce energy with molecular oxygen as the final electron acceptor. Oxidation of fuel molecules describes the removal of electrons from fuel molecules and not the direct combination of molecular oxygen with fuel molecules.
CELLULAR RESPIRATION


Glycolysis
We begin our journey through the stages of respiration with stage II or glycolysis, a nearly universal pathway in living organisms that converts glucose into pyruvic acid. In a series of reactions occurring in the cell cytoplasm, glucose and other 6-carbon monosaccharides are split into 3-carbon molecules of pyruvic acid (see Figure 4.12).

Acetyl-CoA: Strategic Intermediate in Respiration
In aerobic metabolis,m the two molecules of pyruvic acid formed during glycolysis enter a mitochondrion. There, each molecule is oxidized, and one of the carbons is released as carbon dioxide (see Figure 4.13). The 2-carbon residue reacts with coenzyme A (CoA) to form acetyl coenzyme A, or acetyl-CoA, and an NADH molecule is also produced.
Krebs Cycle: Oxidation of Acetyl-CoA
Oxidation of the 2-carbon acetyl group of acetyl-CoA occurs within the mitochondrial matrix in a cyclic sequence called the Krebs cycle.
Pyruvate or Pyruvic Acid?
Pyruvic acid is the undissociated form of the acid: O CH3—C—COOH Under physiological conditions pyruvic acid typically dissociates into pyruvate O (CH3—C—COO–) and H+. It is correct to use either term in describing this and other organic acids (such as lactic acid or lactate) in metabolism.

 Knowt
Knowt
