
2.2 The Water Cycle and Oxygen-Demanding Waste
properties of water
composition of water molecules
water molecules are cohesive, meaning that there are strong forces of attraction between molecules of water
water will remain a liquid over a wide variety of temperatures
it has a high boiling point and a low freezing point
it also has a high heat capacity, so liquid water changes temperature very slowly; it can store large amounts of heat without a change in temperature
it has a high heat of vaporization, so it takes a lot of heat to evaporate liquid water
water is the universal solvent and can dissolve a variety of compounds (eg. nutrients, waste, pollutants)
has high surface tension, meaning that its surface behaves like a membrane (its surface is often similar to that of a solid)
ice is less dense than liquid water — water expands when it freezes
causes for concern with water
too much (flooding, eg. Pakistan)
too little (droughts, eg. California)
too poor of quality (contaminated, eg. Flint)
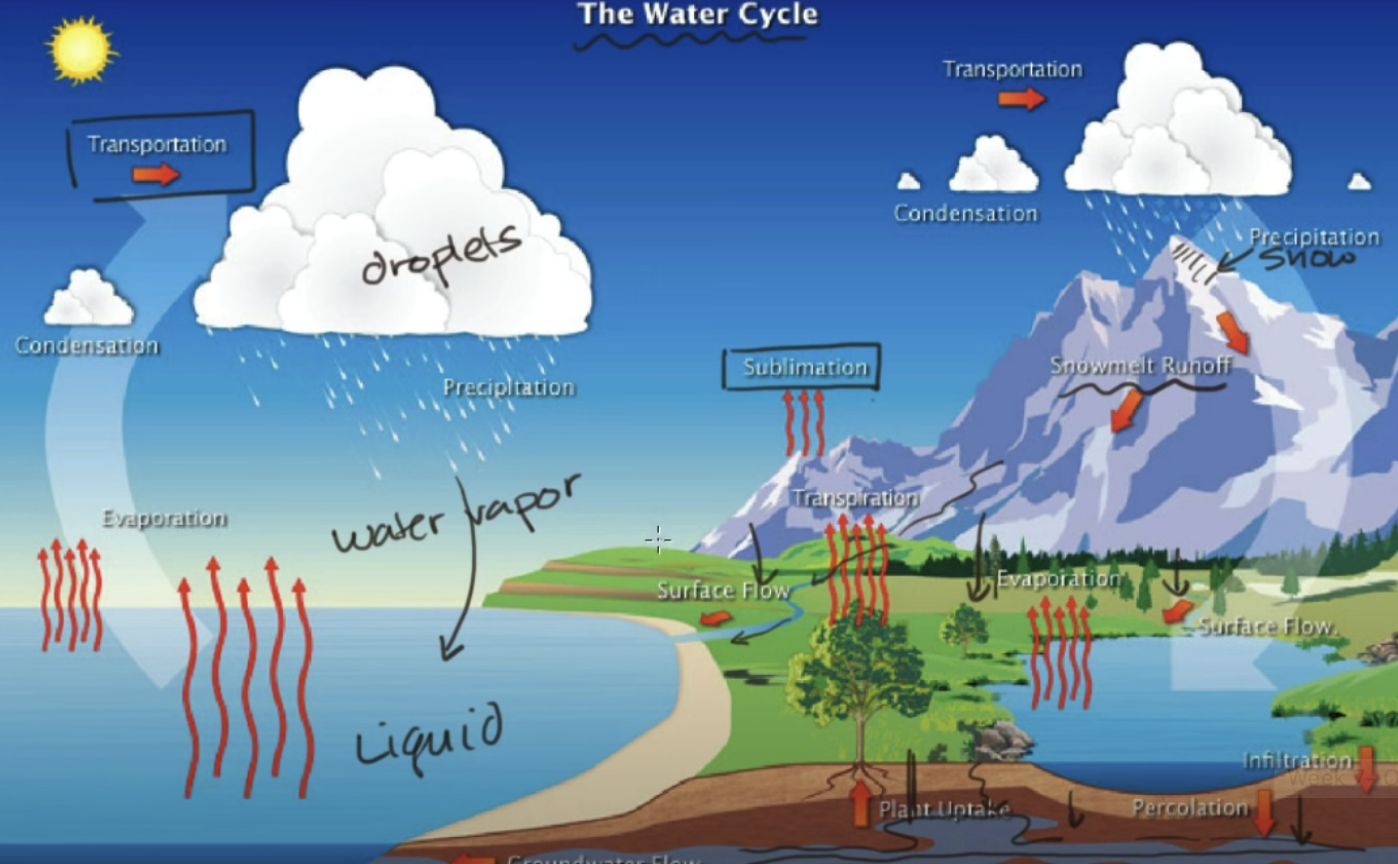
the water cycle
the vast majority of water on earth is saltwater; of the freshwater, most of it is located in glaciers/permanent snow cover and groundwater
 oxygen-demanding waste
oxygen-demanding waste
oxygen demanding waste: organic waste that can be decomposed by aerobic bacteria
aerobic bacteria: bacteria that use oxygen
because the bacteria use so much oxygen, the level of oxygen dissolved (DO) in the water decreases
fish and other animals need high levels of DO to survive
origins of oxygen demanding waste
main cause — failing infrastructure
eg. leaking pipes, failing/overflowing sewage systems
results in the release of oxygen demanding waste, eg. raw sewage, manure, decomposing algae, paper factories, meat-processing plants
streams
stream: a body of water that flows
eg. rivers, creeks
usually recover quickly from oxygen demanding waste, but if they are overloaded, the breakdown of degradable waste by bacteria depletes DO and produces an oxygen sag
oxygen sag: the reduction in dissolved oxygen plotted over a distance along a water body from a point at which sewage or other pollutants have been discharged
biochemical oxygen demand (BOD): the amount of oxygen consumed by bacteria while they decompose organic matter
sediment
a pollutant
sediment/suspended matter: insoluble soil particles which make water turbid
turbid: cloudy, opaque, or thick with suspended matter
2.2 The Water Cycle and Oxygen-Demanding Waste
properties of water
composition of water molecules
water molecules are cohesive, meaning that there are strong forces of attraction between molecules of water
water will remain a liquid over a wide variety of temperatures
it has a high boiling point and a low freezing point
it also has a high heat capacity, so liquid water changes temperature very slowly; it can store large amounts of heat without a change in temperature
it has a high heat of vaporization, so it takes a lot of heat to evaporate liquid water
water is the universal solvent and can dissolve a variety of compounds (eg. nutrients, waste, pollutants)
has high surface tension, meaning that its surface behaves like a membrane (its surface is often similar to that of a solid)
ice is less dense than liquid water — water expands when it freezes
causes for concern with water
too much (flooding, eg. Pakistan)
too little (droughts, eg. California)
too poor of quality (contaminated, eg. Flint)
the water cycle
the vast majority of water on earth is saltwater; of the freshwater, most of it is located in glaciers/permanent snow cover and groundwater
 oxygen-demanding waste
oxygen-demanding waste
oxygen demanding waste: organic waste that can be decomposed by aerobic bacteria
aerobic bacteria: bacteria that use oxygen
because the bacteria use so much oxygen, the level of oxygen dissolved (DO) in the water decreases
fish and other animals need high levels of DO to survive
origins of oxygen demanding waste
main cause — failing infrastructure
eg. leaking pipes, failing/overflowing sewage systems
results in the release of oxygen demanding waste, eg. raw sewage, manure, decomposing algae, paper factories, meat-processing plants
streams
stream: a body of water that flows
eg. rivers, creeks
usually recover quickly from oxygen demanding waste, but if they are overloaded, the breakdown of degradable waste by bacteria depletes DO and produces an oxygen sag
oxygen sag: the reduction in dissolved oxygen plotted over a distance along a water body from a point at which sewage or other pollutants have been discharged
biochemical oxygen demand (BOD): the amount of oxygen consumed by bacteria while they decompose organic matter
sediment
a pollutant
sediment/suspended matter: insoluble soil particles which make water turbid
turbid: cloudy, opaque, or thick with suspended matter
 Knowt
Knowt
