Physics
Thermodynamics:
Study of energy transfer as work and heat
Relationship between heat, temperature, energy, and work
Origin of the word from Greek: "therme" for heat and "dynamis" for force
Temperature on Molecular Level:
Kinetic molecular theory explains temperature
Temperature measures average kinetic energy of atoms and molecules
Higher temperature means more kinetic energy among atoms or molecules
Temperature Measurement:
Different temperature scales and conversions
Kelvin as the unit for absolute temperature
Absolute zero as the lowest energy state
Instrument to Measure Temperature:
Types of thermometers: digital, tympanic, infrared, strip-type, mercury, clinical, laboratory
Everyday Physics Integration: Heat Index
Heat index combines air temperature and humidity
Impact on human body's comfort and cooling mechanisms
High heat index poses risks like cramps, heat exhaustion, and heat stroke
Heat (Energy) and Internal Energy:
Heat is energy in transit between bodies with different temperatures
Heat flows from high to low temperature areas
Internal energy includes both kinetic and potential energy of molecules
Internal energy (U) is the total kinetic and potential energy of particles in a system
Directly proportional to temperature
Higher temperature means more internal energy, lower temperature means less
Comparison between objects A and B
A has 4 times the amount of substance compared to B
Both have the same temperature, so the average kinetic energy of molecules is the same
Object B has more internal energy due to having 4 times the substance
Comparison between two objects
The Venti has 2 times the amount of substance compared to the other
Venti has more internal energy due to the larger amount of substance
Doubling the amount of coffee
Doubling the size does not double the temperature but doubles the internal energy
Internal energy can do work through pressure-volume work by expanding gas
Temperature is a measure of heat energy available to do work in a system
Internal energy can also do work through heat transfer
Summary of quantities
Temperature: average kinetic energy of molecules, basic property of matter
Internal energy (U): total energy of molecules, proportional to temperature
Heat (Q): energy in transit, depends on temperature difference, substances do not contain heat
Practice exercise: temperature change of mercury from -73oC to 723oC
Express temperature change in kelvin and Fahrenheit degrees
Additional Information
Temperature is an intensive property, while internal energy is an extensive property
Internal energy is in Joules, heat is in Joules or Calories
Temperature change can be converted to Kelvin and Fahrenheit degrees
T3.2: Heat and Heat Effects
Terms:
Temperature: Measure of the average kinetic energy (KE) of molecules of an object, indicating how "hot" the object is.
Heat: Total kinetic energy (KE) and potential energy (PE) of the molecules in an object, transferred from one body to another due to a difference in temperature.
Internal Energy (U): Total amount of KE and PE of all the particles in the system.
Heat Capacity: Amount of heat needed to change the temperature of an object by a degree, measured in joules per degree Celsius (J/Kg.C).
Equations/Formulae:
Heat Capacity formula: C = mc, where C is the heat capacity, m is the mass, and c is the specific heat in J/Kg.C.
Specific Heat: The amount of heat needed to change the temperature of a unit mass of a substance by 1 degree.
Specific Latent Heat of Fusion: The amount of thermal energy required to change the state of 1 kg of a substance from solid to liquid or vice versa.
Specific Latent Heat of Vaporization: The amount of thermal energy required to change the state of 1 kg of a substance from liquid to gas or vice versa.
These terms and equations/formulas are used to explain the relationship between temperature, heat, and internal energy, as well as the effects of heat transfer, expansion, and changes in state of matter.
expound on the equations/formulas mentioned in the document:
Heat Capacity Formula:
The heat capacity (C) of an object is given by the formula C = mc, where:
C = heat capacity (in joules per degree Celsius, J/Kg.C)
m = mass of the object (in kilograms, kg)
c = specific heat of the material (in joules per kilogram per degree Celsius, J/Kg.C)
This formula is used to calculate the amount of heat needed to change the temperature of an object by a degree.
Specific Heat:
Specific heat (c) is the amount of heat needed to change the temperature of a unit mass of a substance by 1 degree.
It is a property of the material and is measured in joules per kilogram per degree Celsius (J/Kg.C).
The specific heat of a substance determines how much heat is required to change its temperature.
Specific Latent Heat of Fusion:
The specific latent heat of fusion (L) is the amount of thermal energy required to change the state of 1 kg of a substance from solid to liquid or vice versa.
It is measured in joules per kilogram (J/kg).
This energy is absorbed or released during the phase change from solid to liquid or vice versa.
Specific Latent Heat of Vaporization:
The specific latent heat of vaporization (Lv) is the amount of thermal energy required to change the state of 1 kg of a substance from liquid to gas or vice versa.
It is also measured in joules per kilogram (J/kg).
This energy is absorbed or released during the phase change from liquid to gas or vice versa.
examples:
Heat Capacity Formula: Example: Given:
Mass (m) = 2.0 kg
Specific heat (c) of aluminum = 900 J/kg.C (a) Calculate the heat capacity of aluminum. Solution: Using the formula C = mc, we can calculate the heat capacity: C = (2.0 kg) * (900 J/kg.C) = 1800 J/C Therefore, the heat capacity of the aluminum object is 1800 J/C.
(b) Calculate the amount of heat needed to change the temperature of the object from 25°C to 35°C. Solution: The change in temperature (ΔT) = 35°C - 25°C = 10°C Using the heat capacity (C) calculated in part (a): Heat (Q) = C ΔT Q = 1800 J/C 10°C = 18000 J Therefore, the amount of heat needed to change the temperature of the object from 25°C to 35°C is 18000 J.
Specific Latent Heat of Fusion: Example: Given:
Specific latent heat of fusion (L) for ice = 334,000 J/kg If 1 kg of ice at 0°C is completely melted to water at 0°C, calculate the amount of heat absorbed. Solution: The amount of heat absorbed during the phase change from solid to liquid is given by: Heat (Q) = mass specific latent heat of fusion Q = 1 kg 334,000 J/kg = 334,000 J Therefore, the amount of heat absorbed during the melting of 1 kg of ice is 334,000 J.
Specific Latent Heat of Vaporization: Example: Given:
Specific latent heat of vaporization (Lv) for water = 2,260,000 J/kg If 1 kg of water at 100°C is completely vaporized to steam at 100°C, calculate the amount of heat absorbed. Solution: The amount of heat absorbed during the phase change from liquid to gas is given by: Heat (Q) = mass specific latent heat of vaporization Q = 1 kg 2,260,000 J/kg = 2,260,000 J Therefore, the amount of heat absorbed during the vaporization of 1 kg of water is 2,260,000 J.
T3.3: Conditions Necessary for HeatTransfer to Occur
Terms:
Heat: The energy that flows from one body to another due to a temperature difference.
Conduction: The transfer of heat through a diffusive process wherein molecules transmit their kinetic energy to other molecules by colliding with them.
Convection: The transfer of heat associated with the motion of the medium, such as when a hot material flows into a cold material.
Radiation: The transfer of heat via electromagnetic radiation, such as the radiation from the Sun.
Equations/Formula:
Q = mcΔT: The equation for calculating heat transfer, where Q is the heat transferred, m is the mass, c is the specific heat, and ΔT is the change in temperature.
Specific Heat (c): The specific heat of a substance is the amount of heat required to raise the temperature of a unit mass of the substance by one degree Celsius.
The document also includes sample problems involving specific heat and heat transfer, providing a practical application of the concepts discussed.
The formula Q = mcΔT is used to calculate the amount of heat transferred. Here's an explanation of the formula and some examples:
Explanation of the Formula:
Q represents the amount of heat transferred, measured in joules (J).
m represents the mass of the substance, measured in kilograms (kg) or grams (g).
c represents the specific heat capacity of the substance, measured in joules per kilogram per degree Celsius (J/kg°C) or joules per gram per degree Celsius (J/g°C).
ΔT represents the change in temperature, measured in degrees Celsius (°C) or Kelvin (K).
The formula essentially states that the amount of heat transferred (Q) is equal to the product of the mass (m), the specific heat capacity (c), and the change in temperature (ΔT) of the substance.
Examples:
Example of Using the Formula for Water:
Suppose we have 1 kg (1000 g) of water and we want to raise its temperature by 10°C.
The specific heat capacity of water is approximately 4186 J/kg°C.
Using the formula Q = mcΔT: Q = (1000 g) (4186 J/kg°C) (10°C) Q = 41,860 J
Therefore, it would require 41,860 joules of heat to raise the temperature of 1 kg of water by 10°C.
Example of Using the Formula for a Metal:
Consider a 500 g piece of metal with a specific heat capacity of 500 J/kg°C. If the temperature of the metal increases by 50°C, we can calculate the heat transfer.
Using the formula Q = mcΔT: Q = (500 g) (500 J/kg°C) (50°C) Q = 12,500 J
Therefore, it would require 12,500 joules of heat to raise the temperature of the metal by 50°C
T3.4: Using Heat to do Work
Terms:
Thermodynamics: The study of heat and its transformations to other forms of energy, such as mechanical, electrical, or chemical, and vice versa.
Mechanical Equivalent of Heat: The quantitative relationship between heat and work, established by James Prescott Joules.
Heat Engine: A device that changes heat energy to mechanical work continuously.
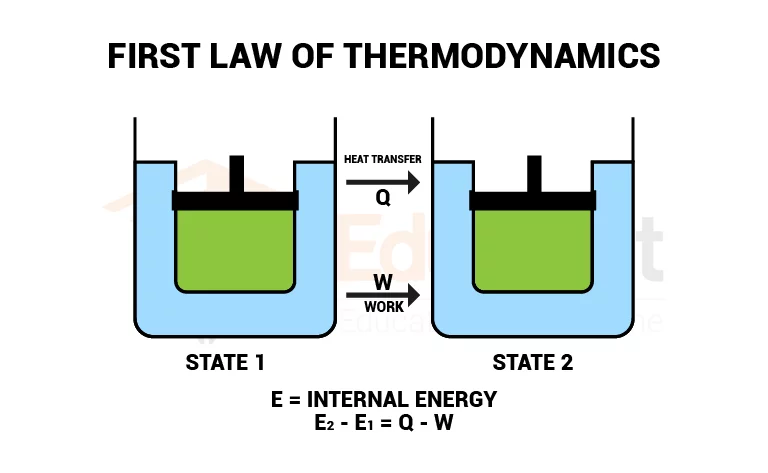
1st Law of Thermodynamics: The law stating that the internal energy of a system is affected by the heat it absorbs (or releases) and the work done by the system or work done on the system.
Equation/Formula:
1st Law of Thermodynamics Equation: ΔU = Q - W
ΔU: Change in internal energy
Q: Heat transferred into the system
W: Work done on or by the system
Relationship between Internal energy, Heat, and Work:
The total increase (or decrease) in the thermal energy of the system is equal to the total work done on (or by) it and the heat added (or removed) to it.
Conversion of Work to Heat: W = JQ
W: Work done
J: Mechanical equivalent of heat
Q: Heat developed
1st Law of Thermodynamics Equation: ΔU = Q - W
ΔU: Change in internal energy
Q: Heat transferred into the system
W: Work done on or by the system
Example: Suppose a closed system absorbs 150 J of heat and does 100 J of work. How much would be the change in internal energy? Solution: ΔU = Q - W ΔU = 150 J - 100 J ΔU = 50 J
Relationship between Internal energy, Heat, and Work:
The total increase (or decrease) in the thermal energy of the system is equal to the total work done on (or by) it and the heat added (or removed) to it.
Example: A closed thermodynamics system absorbs 100 J of heat, resulting in a net-zero change in its internal energy. How much work is involved in this process? Identify whether the work is done on or by the system. Solution: ΔU = 0 Q = 100 J ΔU = 0 W = ? ΔU = Q - W 0 = 100 J - W W = 100 J (work is done by the system)
Conversion of Work to Heat: W = JQ
W: Work done
J: Mechanical equivalent of heat
Q: Heat developed
Example: Suppose that by rubbing your hands, you perform 4500 J of work against friction. How much heat in calories did you generate? Solution: W = 4500 J J = 4.186 J/cal (mechanical equivalent of heat) Q = W / J Q = 4500 J / 4.186 J/cal Q ≈ 1075 cal
Continuation of 3.4
Thermodynamics: The study of the relationships between heat, work, and energy.
Internal Energy: The sum of kinetic and potential energies of a system's particles and their interactions.
Heat: The transfer of thermal energy from a hotter object to a colder object.
Work: The transfer of energy that results in a change in the state of a system.
Heat Engine: A device that converts heat energy into mechanical work continuously.
1st Law of Thermodynamics: States that the total increase or decrease in the thermal energy of a system is equal to the total work done on or by it and the heat added to it.
Equations and Formulas:
1st Law of Thermodynamics Equation: ΔU = Q - W
ΔU: Change in internal energy
Q: Heat added to the system
W: Work done by the system
Specific Heat Equation: Q = mcΔT
Q: Heat added or removed
m: Mass of the substance
c: Specific heat capacity
ΔT: Change in temperature
Mechanical Equivalent of Heat: J = 4.186 J/cal
J: Mechanical energy or work done
cal: Calorie, a unit of heat
Relationship between Internal energy, Heat, and Work:
ΔU = Q - W
(+) Q: Heat transfers INTO the system
(-) Q: Heat transfers OUT of the system
(+) W: Work is DONE BY the system
(-) W: Work is DONE ON the system
Heat Engine Operation:
Thermal energy is introduced into the engine.
Some of this energy is converted to mechanical work.
Some heat (waste heat) is released into the environment at a lower temperature.
1st Law of Thermodynamics Equation: ΔU = Q - W
ΔU: Change in internal energy
Q: Heat added to the system
W: Work done by the system
Example: Suppose a closed system absorbs 150 J of heat and does 100 J of work. How much would be the change in internal energy? Solution: ΔU = Q - W ΔU = 150 J - 100 J ΔU = 50 J Result: The change in internal energy is 50 J.
Specific Heat Equation: Q = mcΔT
Q: Heat added or removed
m: Mass of the substance
c: Specific heat capacity
ΔT: Change in temperature
Example: Calculate the change in temperature of 1.0 kg of water that is initially at room temperature (22°C) if 3.0 kJ of heat is added. Solution: Q = 3000 J (3.0 kJ = 3000 J) m = 1.0 kg c = 4186 J/kg°C (specific heat of water) ΔT = Q / (mc) ΔT = 3000 J / (1.0 kg * 4186 J/kg°C) ΔT ≈ 0.716°C Result: The change in temperature is approximately 0.716°C.
Relationship between Internal energy, Heat, and Work: ΔU = Q - W
ΔU: Change in internal energy
Q: Heat added to the system
W: Work done by the system
Example: A closed thermodynamics system absorbs 100 J of heat, resulting in a net-zero change in its internal energy. How much work is involved in this process? Identify whether the work is done on or by the system. Solution: ΔU = 0 Q = 100 J ΔU = Q - W 0 = 100 J - W W = 100 J Result: 100 J of work is done by the system.
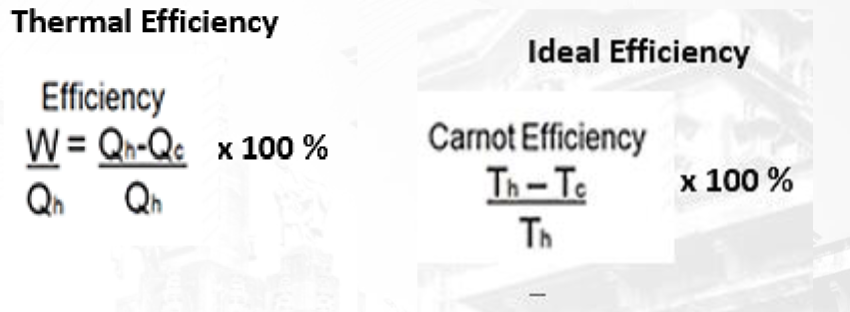
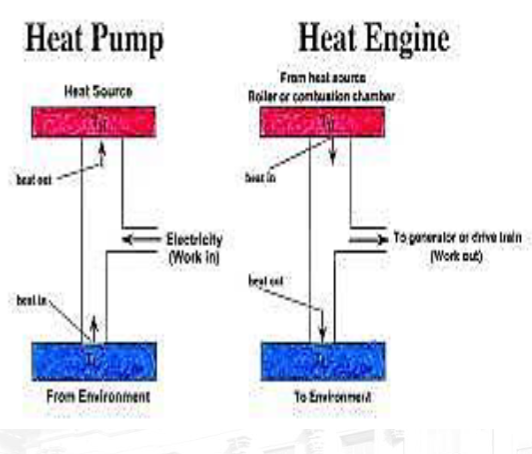
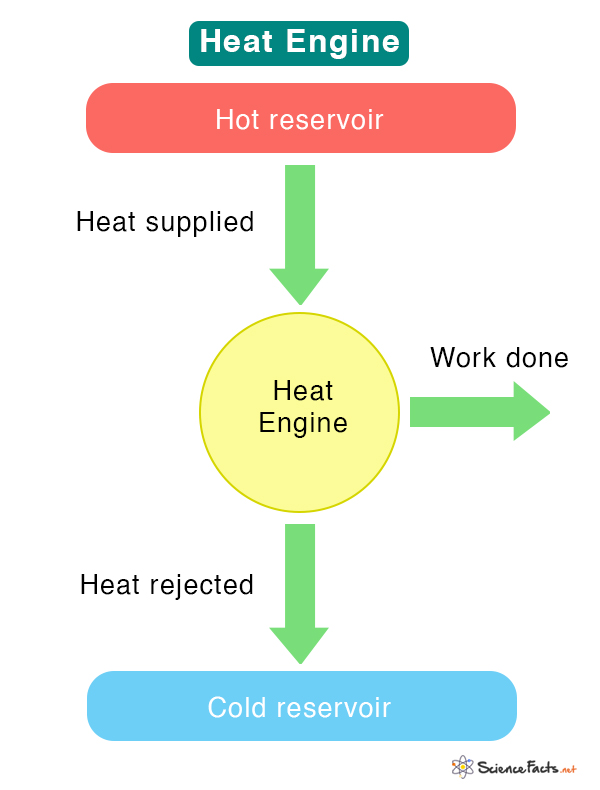
T3.5: Heat Engines and Thermal Efficiency
erms:
Heat Engine: A device that converts thermal energy into other forms of energy, such as mechanical or electrical energy. It is a cyclic device that starts and returns to the same thermodynamic state.
Adiabatic Process: A process in which a gas is compressed or expanded without any heat entering or leaving the system.
Thermal Efficiency: A measure of how well an engine operates, indicating how well a heat engine converts heat into useful work.
Equations/Formula:
Thermal Efficiency (Eff) = (Output energy - mechanical work done) / Input energy (heat QH from the hot reservoir) x 100%
Ideal Efficiency of a Carnot Engine: Eff = (QH - QC) / QH x 100%
Kelvin to Celsius conversion: Kelvin = Celsius + 273.15
Sample Problem 1: An engine takes in 8000 J and discards 4400 J of heat. a) Mechanical work = 3600 J, b) Thermal efficiency = 45%
Sample Problem 2: A heat engine performs 9,200 J of mechanical work while discarding 4500 J to the heat sink. a) Heat absorbed from the hot reservoir, b) Efficiency of the engine.
Thermal Efficiency (Eff) = (Output energy - mechanical work done) / Input energy (heat QH from the hot reservoir) x 100%
This formula measures how well an engine operates by indicating how well a heat engine converts heat into useful work. It compares the output energy (mechanical work done) to the input energy (heat absorbed from the hot reservoir).
Example: If an engine takes in 10,000 J of heat and performs 4,000 J of mechanical work, the thermal efficiency would be: Eff = (10,000 J - 4,000 J) / 10,000 J x 100% = 60%
Ideal Efficiency of a Carnot Engine: Eff = (QH - QC) / QH x 100%
This formula calculates the ideal efficiency of a Carnot engine, which operates between two temperatures, a hot temperature (Th) and a cold temperature (Tc) expressed in Kelvin.
Example: If a Carnot engine absorbs 2000 J of heat from the hot reservoir and discards 800 J to the cold reservoir, the ideal efficiency would be: Eff = (2000 J - 800 J) / 2000 J x 100% = 60%
Kelvin to Celsius conversion: Kelvin = Celsius + 273.15
This formula is used to convert temperatures from Celsius to Kelvin.
Example: If the temperature is 25°C, it can be converted to Kelvin as: Kelvin = 25°C + 273.15 = 298.15 K
Second Law of Thermodynamics: The Second Law of Thermodynamics states that in any energy transfer or transformation, the total entropy of a closed system will always increase over time. This law implies that processes occur in a particular direction and that not all energy can be converted into useful work. It also introduces the concept of irreversibility in natural processes, highlighting limitations on energy conversion efficiency.
Equation of Thermal Efficiency: The thermal efficiency of a system is a measure of how effectively it converts input energy into useful work or output. The equation for thermal efficiency is given by: Efficiency = (Useful output energy / Input energy) * 100%. This formula quantifies the ratio of useful work produced by a system to the total input energy supplied to the system.
Ideal Efficiency: Ideal efficiency refers to the maximum possible efficiency that a system can achieve under ideal conditions, without any energy losses. It represents the theoretical upper limit of efficiency for a given process or device. Achieving ideal efficiency is often impossible in real-world scenarios due to factors such as friction, heat loss, and other inefficiencies.
Heat Pump: A heat pump is a device that transfers heat from a colder space (or source) to a warmer space (or sink) by using mechanical work. Heat pumps operate based on the principles of thermodynamics, specifically the transfer of heat from a low-temperature reservoir to a high-temperature reservoir. They are commonly used for heating or cooling applications in buildings, refrigeration systems, and industrial processes.
T3.6: Electrical Generation, Transmission and Distribution
A heat engine is used to generate electricity
Sources of Electrical Energy: Providing Fuel → Igniting Fuel → Fuel being superheated → Driving the turbine → Generating Electricity
Companies that generate electricity:
National Power Corporation (Napocor)
Philippine National Oil Company-Energy Development Corporation (PNOC-EDC)
Aboitiz Power
First Gen Corporation
A power plant or power station is where electricity is generated
Thermal
Thermal power plants convert thermal energy (heat) into electrical energy. They typically use fossil fuels like coal, natural gas, or oil to generate steam, which drives a turbine connected to a generator to produce electricity.
Kinetic
Kinetic power plants, on the other hand, convert the kinetic energy of moving fluids (such as wind or flowing water) into electrical energy. Wind turbines and hydroelectric power plants are examples of kinetic power plants
Source of energy → turn of turbine → rotation of coils or magnets of generator → production of electricity
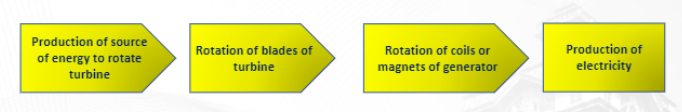
The basic components of electricity generation are:
1. Turbines – shaft with rotating blades
2. Generators – consist of powerful electromagnets surrounded by coils converting mechanical to electrical energy
3. Transformers – a device that lowers or raises the voltage of an(AC)ALTERNATING CURRENT source.
Alternating Current (AC) is a type of electrical current, in which the direction of the flow of electrons switches back and forth at regular intervals or cycles.
Sources of Electrical energy:
Renewable resources – can be replenished naturally
Non-renewable resources – more rapidly used than formed.
Electricity undergoes a series of processes before it reaches us. Electricity is generated in a power plant. Transformed to higher-voltage electricity at substations Sent to the transmission network Reduced and transmitted to the distribution companies. The distributors bring power to millions of consumers. An electrical grid is useful in balancing an electricity transmission system
Electrical generation:
The process of converting mechanical, chemical, or other forms of energy into electrical energy.
Key methods of generation include fossil fuel power plants, nuclear power plants, hydroelectric power plants, solar power, wind power, and geothermal power.
Generation capacity is measured in watts or kilowatts (kW), and the total amount of electricity generated over time is measured in kilowatt-hours (kWh).
Efficiency, capacity factor, and availability are important metrics for evaluating the performance of generation facilities.
Electrical transmission:
The process of moving electricity from power plants to substations or distribution centers over long distances.
High-voltage transmission lines are used to minimize energy losses during transport.
Transformers are used to step up voltage for efficient transmission and step down voltage for distribution.
Grid infrastructure, including substations and control systems, plays a crucial role in managing the flow of electricity across the transmission network.
Electrical distribution:
The final stage of delivering electricity to end-users, including residential, commercial, and industrial customers.
Distribution networks consist of low-voltage lines, transformers, and distribution substations.
Smart grid technologies enable efficient monitoring, control, and optimization of distribution systems.
Reliability, voltage regulation, and power quality are important considerations in distribution system design and operation.
Overall, the integration of generation, transmission, and distribution systems is essential for ensuring a reliable and efficient supply of electricity to meet the needs of consumers and support economic development.
T3.7 Magnetism and T3.8: Induction of Electricity by Magnetism
Protons and electrons are oppositely charged that react to both electric and magnetic fields
No electromagnetic force means atoms and molecules would never form
Electromagnetism is the force exerted between electric charges on one another, and it is a branch of physics dealing with the study of electromagnetic force.
Magnets produce a magnetic field and can attract or repel other materials containing iron.
Ferromagnetic
strongly attracted to magnets
Paramagnetic
Slightly attracted by strong magnets
Diamagnetic
Repelled by strong magnets
Magnetic field lines emerge from the North pole and merge at the South pole of a magnet. → magnetic force (The force exerted by a magnet on another magnet or a magnetic material.)
A region around a magnet where magnetic forces are experienced.
The regions of a magnet where the magnetic field is strongest, typically labeled as the north and south poles, are the magnetic poles
An electric current creates a magnetic field in the surrounding space, leading to the discovery of electromagnets.
Electromagnets are magnets that run on electricity and can be turned on and off by changing the amount of electric current that flows through them.
A temporary magnet created by passing an electric current through a coil of wire wrapped around a magnetic material.
Due to the Earth’s Magnetosphere, this allows compasses to work and protect us from radiation from cosmic rays
Magnetic domain: A region within a magnetic material where the magnetic moments of atoms are aligned in the same direction.
Key Terms with Explanation:
Electromagnetism: The force exerted between electric charges on one another, and a branch of physics dealing with the study of electromagnetic force.
Magnetic field: The area around a magnet that has a magnetic force, with field lines emerging from the North pole and merging at the South pole.
Magnetic flux: The number of magnetic field lines per area, indicating the strength of the magnet.
Electromagnets: Magnets that run on electricity and can be turned on and off by changing the amount of electric current that flows through them.
Increase power: increase the current, add loops on the solenoid
Induction of electricity:
Electromagnetic induction: The process of generating an electromotive force (emf) or voltage in a conductor by changing the magnetic field around it.
Faraday's law: States that the induced emf in a circuit is directly proportional to the rate of change of magnetic flux through the circuit.
Lenz's law: States that the direction of the induced current in a circuit is such that it opposes the change in magnetic flux that produced it.
Transformer: A device that uses electromagnetic induction to transfer electrical energy from one circuit to another through a changing magnetic field.
Induction coil: A device that uses electromagnetic induction to produce high-voltage pulses for applications such as spark plugs in internal combustion engines.
A magnetic field around the wire coil is created every time an electric current flows through a wire

Terms and Explanations:
Electromagnetic Induction: The process of generating an electromotive force (emf) in a closed circuit by the relative motion of a magnet and a conductor, leading to the production of an induced current.
Electromagnet: A magnet that runs on electricity, created by passing an electric current through a wire coil to produce a magnetic field.
Electric Motor: A device that converts electrical energy into mechanical energy by utilizing the interaction between electricity and magnetism to produce motion.
Generator: An electrical device that uses the principle of electromagnetic induction to convert mechanical energy into electrical energy by moving a wire near a magnet to create a flow of current.
Transformer: An electrical device that uses electromagnetic induction to transfer energy from one electric circuit to another, often changing the voltage and electric current.
T3.9: Types of Charges
Summary of Terms:
Electric Charge: An electrical property of matter that creates a force between objects. It can be positive, negative, or neutral (no net charge).
Electrostatics: The study of electric charges at rest under the influence of electric forces.
Grounding (Earthing): Allowing a path for charges to flow between a charged object and the earth, removing or balancing its excess charge.
Lightning Rods: Devices that provide a path for electrons in the air to flow from the top of a building to the earth, protecting the building from lightning damage.
Explanation:
Electric Charge: It is an inherent property of matter that results in the attraction or repulsion between objects. It can be positive, negative, or neutral, and it creates an electric force between charged objects.
objects are neutral at nature
Lose electrons = positive charge
Gain electrons = negative charge
Law of electric charges: Unlike attracts, like repels
Electrostatics: This is the branch of physics that deals with the interactions of static (non-moving) electric charges, involving forces between charged objects.
Law of Conservation of Charge
Charges cannot be created nor destroyed but can be transferred from one material to another. Total change in system is constant.
Electrons are the one freely moving
Charging processes:
Friction
Objects with less affinity for electrons will lose electrons (becomes positively charge) to one with greater affinity. Less affinity means it can easily lose electrons and gain less electrons but higher affinity means it will lose less electrons but will gain more electrons.
Conduction
Transfer of electrons by direct contact
When two objects with different electric potentials come into contact, electrons can move from the object with a higher potential to the object with a lower potential.
Induction
Polarization means to induce a charge
rearrangement of electrons within a neutral object when it is brought near a charged object, without direct contact between the two objects.
It is important to note that the neutral object does not actually gain or lose electrons during the induction process.
Grounding (Earthing): This is a safety measure that allows excess charges on an object to flow to the earth, preventing electric shocks and balancing the charge.
Lightning Rods: These are devices used to protect buildings from lightning damage by providing a path for the flow of electrons from the top of the building to the earth, preventing electric surges, fires, and explosions.
T3.10: Ohm’s Law
Complete Circuit
Current can flow all the way around
An electric circuit is a complete closed path through which electric charges can flow
parts of circuit:
Energy source
Electrical conductor
Load
Ohm's law states that the current flowing through a conductor is directly proportional to the voltage applied across it, and inversely proportional to the resistance of the conductor.
Key terms:
Current (I) - The flow of electric charge through a conductor, measured in amperes (A).
Voltage (V) - The electrical potential difference between two points in a circuit, measured in volts (V).
Resistance (R) - The opposition to the flow of electric current in a conductor, measured in ohms (Ω).
Example: If a circuit has a voltage of 12 volts applied across a resistor with a resistance of 4 ohms, the current flowing through the resistor can be calculated using Ohm's law: I = V/R I = 12V / 4Ω I = 3A. Therefore, the current flowing through the resistor is 3 amperes.
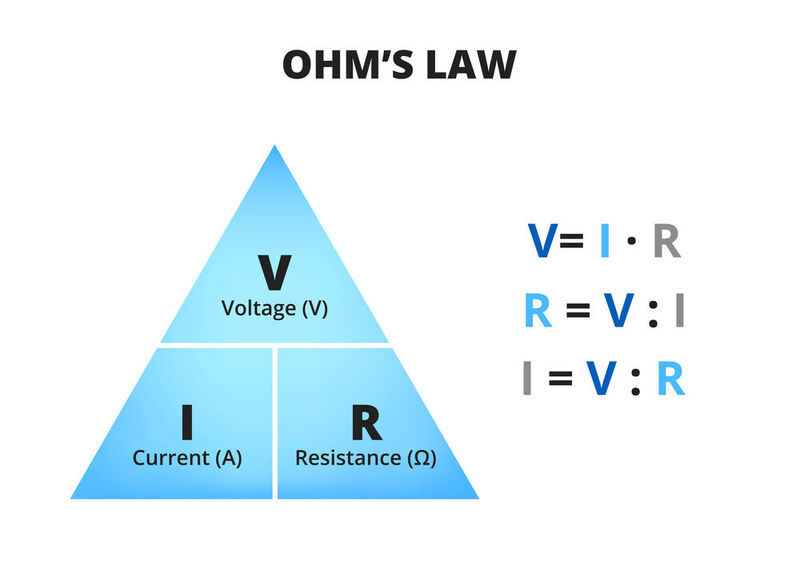
Table of Vocabulary:
A conductor is a material that allows the flow of electric current through it. Conductors have a high density of free electrons that are able to move easily in response to an applied electric field.
Voltage is the force that drives electric current through a conductor.
Physics
Thermodynamics:
Study of energy transfer as work and heat
Relationship between heat, temperature, energy, and work
Origin of the word from Greek: "therme" for heat and "dynamis" for force
Temperature on Molecular Level:
Kinetic molecular theory explains temperature
Temperature measures average kinetic energy of atoms and molecules
Higher temperature means more kinetic energy among atoms or molecules
Temperature Measurement:
Different temperature scales and conversions
Kelvin as the unit for absolute temperature
Absolute zero as the lowest energy state
Instrument to Measure Temperature:
Types of thermometers: digital, tympanic, infrared, strip-type, mercury, clinical, laboratory
Everyday Physics Integration: Heat Index
Heat index combines air temperature and humidity
Impact on human body's comfort and cooling mechanisms
High heat index poses risks like cramps, heat exhaustion, and heat stroke
Heat (Energy) and Internal Energy:
Heat is energy in transit between bodies with different temperatures
Heat flows from high to low temperature areas
Internal energy includes both kinetic and potential energy of molecules
Internal energy (U) is the total kinetic and potential energy of particles in a system
Directly proportional to temperature
Higher temperature means more internal energy, lower temperature means less
Comparison between objects A and B
A has 4 times the amount of substance compared to B
Both have the same temperature, so the average kinetic energy of molecules is the same
Object B has more internal energy due to having 4 times the substance
Comparison between two objects
The Venti has 2 times the amount of substance compared to the other
Venti has more internal energy due to the larger amount of substance
Doubling the amount of coffee
Doubling the size does not double the temperature but doubles the internal energy
Internal energy can do work through pressure-volume work by expanding gas
Temperature is a measure of heat energy available to do work in a system
Internal energy can also do work through heat transfer
Summary of quantities
Temperature: average kinetic energy of molecules, basic property of matter
Internal energy (U): total energy of molecules, proportional to temperature
Heat (Q): energy in transit, depends on temperature difference, substances do not contain heat
Practice exercise: temperature change of mercury from -73oC to 723oC
Express temperature change in kelvin and Fahrenheit degrees
Additional Information
Temperature is an intensive property, while internal energy is an extensive property
Internal energy is in Joules, heat is in Joules or Calories
Temperature change can be converted to Kelvin and Fahrenheit degrees
T3.2: Heat and Heat Effects
Terms:
Temperature: Measure of the average kinetic energy (KE) of molecules of an object, indicating how "hot" the object is.
Heat: Total kinetic energy (KE) and potential energy (PE) of the molecules in an object, transferred from one body to another due to a difference in temperature.
Internal Energy (U): Total amount of KE and PE of all the particles in the system.
Heat Capacity: Amount of heat needed to change the temperature of an object by a degree, measured in joules per degree Celsius (J/Kg.C).
Equations/Formulae:
Heat Capacity formula: C = mc, where C is the heat capacity, m is the mass, and c is the specific heat in J/Kg.C.
Specific Heat: The amount of heat needed to change the temperature of a unit mass of a substance by 1 degree.
Specific Latent Heat of Fusion: The amount of thermal energy required to change the state of 1 kg of a substance from solid to liquid or vice versa.
Specific Latent Heat of Vaporization: The amount of thermal energy required to change the state of 1 kg of a substance from liquid to gas or vice versa.
These terms and equations/formulas are used to explain the relationship between temperature, heat, and internal energy, as well as the effects of heat transfer, expansion, and changes in state of matter.
expound on the equations/formulas mentioned in the document:
Heat Capacity Formula:
The heat capacity (C) of an object is given by the formula C = mc, where:
C = heat capacity (in joules per degree Celsius, J/Kg.C)
m = mass of the object (in kilograms, kg)
c = specific heat of the material (in joules per kilogram per degree Celsius, J/Kg.C)
This formula is used to calculate the amount of heat needed to change the temperature of an object by a degree.
Specific Heat:
Specific heat (c) is the amount of heat needed to change the temperature of a unit mass of a substance by 1 degree.
It is a property of the material and is measured in joules per kilogram per degree Celsius (J/Kg.C).
The specific heat of a substance determines how much heat is required to change its temperature.
Specific Latent Heat of Fusion:
The specific latent heat of fusion (L) is the amount of thermal energy required to change the state of 1 kg of a substance from solid to liquid or vice versa.
It is measured in joules per kilogram (J/kg).
This energy is absorbed or released during the phase change from solid to liquid or vice versa.
Specific Latent Heat of Vaporization:
The specific latent heat of vaporization (Lv) is the amount of thermal energy required to change the state of 1 kg of a substance from liquid to gas or vice versa.
It is also measured in joules per kilogram (J/kg).
This energy is absorbed or released during the phase change from liquid to gas or vice versa.
examples:
Heat Capacity Formula: Example: Given:
Mass (m) = 2.0 kg
Specific heat (c) of aluminum = 900 J/kg.C (a) Calculate the heat capacity of aluminum. Solution: Using the formula C = mc, we can calculate the heat capacity: C = (2.0 kg) * (900 J/kg.C) = 1800 J/C Therefore, the heat capacity of the aluminum object is 1800 J/C.
(b) Calculate the amount of heat needed to change the temperature of the object from 25°C to 35°C. Solution: The change in temperature (ΔT) = 35°C - 25°C = 10°C Using the heat capacity (C) calculated in part (a): Heat (Q) = C ΔT Q = 1800 J/C 10°C = 18000 J Therefore, the amount of heat needed to change the temperature of the object from 25°C to 35°C is 18000 J.
Specific Latent Heat of Fusion: Example: Given:
Specific latent heat of fusion (L) for ice = 334,000 J/kg If 1 kg of ice at 0°C is completely melted to water at 0°C, calculate the amount of heat absorbed. Solution: The amount of heat absorbed during the phase change from solid to liquid is given by: Heat (Q) = mass specific latent heat of fusion Q = 1 kg 334,000 J/kg = 334,000 J Therefore, the amount of heat absorbed during the melting of 1 kg of ice is 334,000 J.
Specific Latent Heat of Vaporization: Example: Given:
Specific latent heat of vaporization (Lv) for water = 2,260,000 J/kg If 1 kg of water at 100°C is completely vaporized to steam at 100°C, calculate the amount of heat absorbed. Solution: The amount of heat absorbed during the phase change from liquid to gas is given by: Heat (Q) = mass specific latent heat of vaporization Q = 1 kg 2,260,000 J/kg = 2,260,000 J Therefore, the amount of heat absorbed during the vaporization of 1 kg of water is 2,260,000 J.
T3.3: Conditions Necessary for HeatTransfer to Occur
Terms:
Heat: The energy that flows from one body to another due to a temperature difference.
Conduction: The transfer of heat through a diffusive process wherein molecules transmit their kinetic energy to other molecules by colliding with them.
Convection: The transfer of heat associated with the motion of the medium, such as when a hot material flows into a cold material.
Radiation: The transfer of heat via electromagnetic radiation, such as the radiation from the Sun.
Equations/Formula:
Q = mcΔT: The equation for calculating heat transfer, where Q is the heat transferred, m is the mass, c is the specific heat, and ΔT is the change in temperature.
Specific Heat (c): The specific heat of a substance is the amount of heat required to raise the temperature of a unit mass of the substance by one degree Celsius.
The document also includes sample problems involving specific heat and heat transfer, providing a practical application of the concepts discussed.
The formula Q = mcΔT is used to calculate the amount of heat transferred. Here's an explanation of the formula and some examples:
Explanation of the Formula:
Q represents the amount of heat transferred, measured in joules (J).
m represents the mass of the substance, measured in kilograms (kg) or grams (g).
c represents the specific heat capacity of the substance, measured in joules per kilogram per degree Celsius (J/kg°C) or joules per gram per degree Celsius (J/g°C).
ΔT represents the change in temperature, measured in degrees Celsius (°C) or Kelvin (K).
The formula essentially states that the amount of heat transferred (Q) is equal to the product of the mass (m), the specific heat capacity (c), and the change in temperature (ΔT) of the substance.
Examples:
Example of Using the Formula for Water:
Suppose we have 1 kg (1000 g) of water and we want to raise its temperature by 10°C.
The specific heat capacity of water is approximately 4186 J/kg°C.
Using the formula Q = mcΔT: Q = (1000 g) (4186 J/kg°C) (10°C) Q = 41,860 J
Therefore, it would require 41,860 joules of heat to raise the temperature of 1 kg of water by 10°C.
Example of Using the Formula for a Metal:
Consider a 500 g piece of metal with a specific heat capacity of 500 J/kg°C. If the temperature of the metal increases by 50°C, we can calculate the heat transfer.
Using the formula Q = mcΔT: Q = (500 g) (500 J/kg°C) (50°C) Q = 12,500 J
Therefore, it would require 12,500 joules of heat to raise the temperature of the metal by 50°C
T3.4: Using Heat to do Work
Terms:
Thermodynamics: The study of heat and its transformations to other forms of energy, such as mechanical, electrical, or chemical, and vice versa.
Mechanical Equivalent of Heat: The quantitative relationship between heat and work, established by James Prescott Joules.
Heat Engine: A device that changes heat energy to mechanical work continuously.
1st Law of Thermodynamics: The law stating that the internal energy of a system is affected by the heat it absorbs (or releases) and the work done by the system or work done on the system.

Equation/Formula:
1st Law of Thermodynamics Equation: ΔU = Q - W
ΔU: Change in internal energy
Q: Heat transferred into the system
W: Work done on or by the system
Relationship between Internal energy, Heat, and Work:
The total increase (or decrease) in the thermal energy of the system is equal to the total work done on (or by) it and the heat added (or removed) to it.
Conversion of Work to Heat: W = JQ
W: Work done
J: Mechanical equivalent of heat
Q: Heat developed
1st Law of Thermodynamics Equation: ΔU = Q - W
ΔU: Change in internal energy
Q: Heat transferred into the system
W: Work done on or by the system
Example: Suppose a closed system absorbs 150 J of heat and does 100 J of work. How much would be the change in internal energy? Solution: ΔU = Q - W ΔU = 150 J - 100 J ΔU = 50 J
Relationship between Internal energy, Heat, and Work:
The total increase (or decrease) in the thermal energy of the system is equal to the total work done on (or by) it and the heat added (or removed) to it.
Example: A closed thermodynamics system absorbs 100 J of heat, resulting in a net-zero change in its internal energy. How much work is involved in this process? Identify whether the work is done on or by the system. Solution: ΔU = 0 Q = 100 J ΔU = 0 W = ? ΔU = Q - W 0 = 100 J - W W = 100 J (work is done by the system)
Conversion of Work to Heat: W = JQ
W: Work done
J: Mechanical equivalent of heat
Q: Heat developed
Example: Suppose that by rubbing your hands, you perform 4500 J of work against friction. How much heat in calories did you generate? Solution: W = 4500 J J = 4.186 J/cal (mechanical equivalent of heat) Q = W / J Q = 4500 J / 4.186 J/cal Q ≈ 1075 cal
Continuation of 3.4
Thermodynamics: The study of the relationships between heat, work, and energy.
Internal Energy: The sum of kinetic and potential energies of a system's particles and their interactions.
Heat: The transfer of thermal energy from a hotter object to a colder object.
Work: The transfer of energy that results in a change in the state of a system.
Heat Engine: A device that converts heat energy into mechanical work continuously.
1st Law of Thermodynamics: States that the total increase or decrease in the thermal energy of a system is equal to the total work done on or by it and the heat added to it.
Equations and Formulas:
1st Law of Thermodynamics Equation: ΔU = Q - W
ΔU: Change in internal energy
Q: Heat added to the system
W: Work done by the system
Specific Heat Equation: Q = mcΔT
Q: Heat added or removed
m: Mass of the substance
c: Specific heat capacity
ΔT: Change in temperature
Mechanical Equivalent of Heat: J = 4.186 J/cal
J: Mechanical energy or work done
cal: Calorie, a unit of heat
Relationship between Internal energy, Heat, and Work:
ΔU = Q - W
(+) Q: Heat transfers INTO the system
(-) Q: Heat transfers OUT of the system
(+) W: Work is DONE BY the system
(-) W: Work is DONE ON the system
Heat Engine Operation:
Thermal energy is introduced into the engine.
Some of this energy is converted to mechanical work.
Some heat (waste heat) is released into the environment at a lower temperature.
1st Law of Thermodynamics Equation: ΔU = Q - W
ΔU: Change in internal energy
Q: Heat added to the system
W: Work done by the system
Example: Suppose a closed system absorbs 150 J of heat and does 100 J of work. How much would be the change in internal energy? Solution: ΔU = Q - W ΔU = 150 J - 100 J ΔU = 50 J Result: The change in internal energy is 50 J.
Specific Heat Equation: Q = mcΔT
Q: Heat added or removed
m: Mass of the substance
c: Specific heat capacity
ΔT: Change in temperature
Example: Calculate the change in temperature of 1.0 kg of water that is initially at room temperature (22°C) if 3.0 kJ of heat is added. Solution: Q = 3000 J (3.0 kJ = 3000 J) m = 1.0 kg c = 4186 J/kg°C (specific heat of water) ΔT = Q / (mc) ΔT = 3000 J / (1.0 kg * 4186 J/kg°C) ΔT ≈ 0.716°C Result: The change in temperature is approximately 0.716°C.
Relationship between Internal energy, Heat, and Work: ΔU = Q - W
ΔU: Change in internal energy
Q: Heat added to the system
W: Work done by the system
Example: A closed thermodynamics system absorbs 100 J of heat, resulting in a net-zero change in its internal energy. How much work is involved in this process? Identify whether the work is done on or by the system. Solution: ΔU = 0 Q = 100 J ΔU = Q - W 0 = 100 J - W W = 100 J Result: 100 J of work is done by the system.
T3.5: Heat Engines and Thermal Efficiency
erms:
Heat Engine: A device that converts thermal energy into other forms of energy, such as mechanical or electrical energy. It is a cyclic device that starts and returns to the same thermodynamic state.

Adiabatic Process: A process in which a gas is compressed or expanded without any heat entering or leaving the system.
Thermal Efficiency: A measure of how well an engine operates, indicating how well a heat engine converts heat into useful work.
Equations/Formula:
Thermal Efficiency (Eff) = (Output energy - mechanical work done) / Input energy (heat QH from the hot reservoir) x 100%
Ideal Efficiency of a Carnot Engine: Eff = (QH - QC) / QH x 100%
Kelvin to Celsius conversion: Kelvin = Celsius + 273.15
Sample Problem 1: An engine takes in 8000 J and discards 4400 J of heat. a) Mechanical work = 3600 J, b) Thermal efficiency = 45%
Sample Problem 2: A heat engine performs 9,200 J of mechanical work while discarding 4500 J to the heat sink. a) Heat absorbed from the hot reservoir, b) Efficiency of the engine.
Thermal Efficiency (Eff) = (Output energy - mechanical work done) / Input energy (heat QH from the hot reservoir) x 100%
This formula measures how well an engine operates by indicating how well a heat engine converts heat into useful work. It compares the output energy (mechanical work done) to the input energy (heat absorbed from the hot reservoir).
Example: If an engine takes in 10,000 J of heat and performs 4,000 J of mechanical work, the thermal efficiency would be: Eff = (10,000 J - 4,000 J) / 10,000 J x 100% = 60%
Ideal Efficiency of a Carnot Engine: Eff = (QH - QC) / QH x 100%
This formula calculates the ideal efficiency of a Carnot engine, which operates between two temperatures, a hot temperature (Th) and a cold temperature (Tc) expressed in Kelvin.
Example: If a Carnot engine absorbs 2000 J of heat from the hot reservoir and discards 800 J to the cold reservoir, the ideal efficiency would be: Eff = (2000 J - 800 J) / 2000 J x 100% = 60%
Kelvin to Celsius conversion: Kelvin = Celsius + 273.15
This formula is used to convert temperatures from Celsius to Kelvin.
Example: If the temperature is 25°C, it can be converted to Kelvin as: Kelvin = 25°C + 273.15 = 298.15 K
Second Law of Thermodynamics: The Second Law of Thermodynamics states that in any energy transfer or transformation, the total entropy of a closed system will always increase over time. This law implies that processes occur in a particular direction and that not all energy can be converted into useful work. It also introduces the concept of irreversibility in natural processes, highlighting limitations on energy conversion efficiency.
Equation of Thermal Efficiency: The thermal efficiency of a system is a measure of how effectively it converts input energy into useful work or output. The equation for thermal efficiency is given by: Efficiency = (Useful output energy / Input energy) * 100%. This formula quantifies the ratio of useful work produced by a system to the total input energy supplied to the system.
Ideal Efficiency: Ideal efficiency refers to the maximum possible efficiency that a system can achieve under ideal conditions, without any energy losses. It represents the theoretical upper limit of efficiency for a given process or device. Achieving ideal efficiency is often impossible in real-world scenarios due to factors such as friction, heat loss, and other inefficiencies.
Heat Pump: A heat pump is a device that transfers heat from a colder space (or source) to a warmer space (or sink) by using mechanical work. Heat pumps operate based on the principles of thermodynamics, specifically the transfer of heat from a low-temperature reservoir to a high-temperature reservoir. They are commonly used for heating or cooling applications in buildings, refrigeration systems, and industrial processes.
T3.6: Electrical Generation, Transmission and Distribution
A heat engine is used to generate electricity
Sources of Electrical Energy: Providing Fuel → Igniting Fuel → Fuel being superheated → Driving the turbine → Generating Electricity
Companies that generate electricity:
National Power Corporation (Napocor)
Philippine National Oil Company-Energy Development Corporation (PNOC-EDC)
Aboitiz Power
First Gen Corporation
A power plant or power station is where electricity is generated
Thermal
Thermal power plants convert thermal energy (heat) into electrical energy. They typically use fossil fuels like coal, natural gas, or oil to generate steam, which drives a turbine connected to a generator to produce electricity.
Kinetic
Kinetic power plants, on the other hand, convert the kinetic energy of moving fluids (such as wind or flowing water) into electrical energy. Wind turbines and hydroelectric power plants are examples of kinetic power plants
Source of energy → turn of turbine → rotation of coils or magnets of generator → production of electricity

The basic components of electricity generation are:
1. Turbines – shaft with rotating blades
2. Generators – consist of powerful electromagnets surrounded by coils converting mechanical to electrical energy
3. Transformers – a device that lowers or raises the voltage of an(AC)ALTERNATING CURRENT source.
Alternating Current (AC) is a type of electrical current, in which the direction of the flow of electrons switches back and forth at regular intervals or cycles.
Sources of Electrical energy:
Renewable resources – can be replenished naturally
Non-renewable resources – more rapidly used than formed.
Electricity undergoes a series of processes before it reaches us. Electricity is generated in a power plant. Transformed to higher-voltage electricity at substations Sent to the transmission network Reduced and transmitted to the distribution companies. The distributors bring power to millions of consumers. An electrical grid is useful in balancing an electricity transmission system
Electrical generation:
The process of converting mechanical, chemical, or other forms of energy into electrical energy.
Key methods of generation include fossil fuel power plants, nuclear power plants, hydroelectric power plants, solar power, wind power, and geothermal power.
Generation capacity is measured in watts or kilowatts (kW), and the total amount of electricity generated over time is measured in kilowatt-hours (kWh).
Efficiency, capacity factor, and availability are important metrics for evaluating the performance of generation facilities.
Electrical transmission:
The process of moving electricity from power plants to substations or distribution centers over long distances.
High-voltage transmission lines are used to minimize energy losses during transport.
Transformers are used to step up voltage for efficient transmission and step down voltage for distribution.
Grid infrastructure, including substations and control systems, plays a crucial role in managing the flow of electricity across the transmission network.
Electrical distribution:
The final stage of delivering electricity to end-users, including residential, commercial, and industrial customers.
Distribution networks consist of low-voltage lines, transformers, and distribution substations.
Smart grid technologies enable efficient monitoring, control, and optimization of distribution systems.
Reliability, voltage regulation, and power quality are important considerations in distribution system design and operation.
Overall, the integration of generation, transmission, and distribution systems is essential for ensuring a reliable and efficient supply of electricity to meet the needs of consumers and support economic development.
T3.7 Magnetism and T3.8: Induction of Electricity by Magnetism
Protons and electrons are oppositely charged that react to both electric and magnetic fields
No electromagnetic force means atoms and molecules would never form
Electromagnetism is the force exerted between electric charges on one another, and it is a branch of physics dealing with the study of electromagnetic force.
Magnets produce a magnetic field and can attract or repel other materials containing iron.
Ferromagnetic
strongly attracted to magnets
Paramagnetic
Slightly attracted by strong magnets
Diamagnetic
Repelled by strong magnets
Magnetic field lines emerge from the North pole and merge at the South pole of a magnet. → magnetic force (The force exerted by a magnet on another magnet or a magnetic material.)
A region around a magnet where magnetic forces are experienced.
The regions of a magnet where the magnetic field is strongest, typically labeled as the north and south poles, are the magnetic poles
An electric current creates a magnetic field in the surrounding space, leading to the discovery of electromagnets.
Electromagnets are magnets that run on electricity and can be turned on and off by changing the amount of electric current that flows through them.
A temporary magnet created by passing an electric current through a coil of wire wrapped around a magnetic material.
Due to the Earth’s Magnetosphere, this allows compasses to work and protect us from radiation from cosmic rays
Magnetic domain: A region within a magnetic material where the magnetic moments of atoms are aligned in the same direction.
Key Terms with Explanation:
Electromagnetism: The force exerted between electric charges on one another, and a branch of physics dealing with the study of electromagnetic force.
Magnetic field: The area around a magnet that has a magnetic force, with field lines emerging from the North pole and merging at the South pole.
Magnetic flux: The number of magnetic field lines per area, indicating the strength of the magnet.
Electromagnets: Magnets that run on electricity and can be turned on and off by changing the amount of electric current that flows through them.
Increase power: increase the current, add loops on the solenoid
Induction of electricity:
Electromagnetic induction: The process of generating an electromotive force (emf) or voltage in a conductor by changing the magnetic field around it.
Faraday's law: States that the induced emf in a circuit is directly proportional to the rate of change of magnetic flux through the circuit.
Lenz's law: States that the direction of the induced current in a circuit is such that it opposes the change in magnetic flux that produced it.
Transformer: A device that uses electromagnetic induction to transfer electrical energy from one circuit to another through a changing magnetic field.
Induction coil: A device that uses electromagnetic induction to produce high-voltage pulses for applications such as spark plugs in internal combustion engines.
A magnetic field around the wire coil is created every time an electric current flows through a wire

Terms and Explanations:
Electromagnetic Induction: The process of generating an electromotive force (emf) in a closed circuit by the relative motion of a magnet and a conductor, leading to the production of an induced current.
Electromagnet: A magnet that runs on electricity, created by passing an electric current through a wire coil to produce a magnetic field.
Electric Motor: A device that converts electrical energy into mechanical energy by utilizing the interaction between electricity and magnetism to produce motion.
Generator: An electrical device that uses the principle of electromagnetic induction to convert mechanical energy into electrical energy by moving a wire near a magnet to create a flow of current.
Transformer: An electrical device that uses electromagnetic induction to transfer energy from one electric circuit to another, often changing the voltage and electric current.
T3.9: Types of Charges
Summary of Terms:
Electric Charge: An electrical property of matter that creates a force between objects. It can be positive, negative, or neutral (no net charge).
Electrostatics: The study of electric charges at rest under the influence of electric forces.
Grounding (Earthing): Allowing a path for charges to flow between a charged object and the earth, removing or balancing its excess charge.
Lightning Rods: Devices that provide a path for electrons in the air to flow from the top of a building to the earth, protecting the building from lightning damage.
Explanation:
Electric Charge: It is an inherent property of matter that results in the attraction or repulsion between objects. It can be positive, negative, or neutral, and it creates an electric force between charged objects.
objects are neutral at nature
Lose electrons = positive charge
Gain electrons = negative charge
Law of electric charges: Unlike attracts, like repels
Electrostatics: This is the branch of physics that deals with the interactions of static (non-moving) electric charges, involving forces between charged objects.
Law of Conservation of Charge
Charges cannot be created nor destroyed but can be transferred from one material to another. Total change in system is constant.
Electrons are the one freely moving
Charging processes:
Friction
Objects with less affinity for electrons will lose electrons (becomes positively charge) to one with greater affinity. Less affinity means it can easily lose electrons and gain less electrons but higher affinity means it will lose less electrons but will gain more electrons.
Conduction
Transfer of electrons by direct contact
When two objects with different electric potentials come into contact, electrons can move from the object with a higher potential to the object with a lower potential.
Induction
Polarization means to induce a charge
rearrangement of electrons within a neutral object when it is brought near a charged object, without direct contact between the two objects.
It is important to note that the neutral object does not actually gain or lose electrons during the induction process.
Grounding (Earthing): This is a safety measure that allows excess charges on an object to flow to the earth, preventing electric shocks and balancing the charge.
Lightning Rods: These are devices used to protect buildings from lightning damage by providing a path for the flow of electrons from the top of the building to the earth, preventing electric surges, fires, and explosions.
T3.10: Ohm’s Law
Complete Circuit
Current can flow all the way around
An electric circuit is a complete closed path through which electric charges can flow
parts of circuit:
Energy source
Electrical conductor
Load

Ohm's law states that the current flowing through a conductor is directly proportional to the voltage applied across it, and inversely proportional to the resistance of the conductor.
Key terms:
Current (I) - The flow of electric charge through a conductor, measured in amperes (A).
Voltage (V) - The electrical potential difference between two points in a circuit, measured in volts (V).
Resistance (R) - The opposition to the flow of electric current in a conductor, measured in ohms (Ω).
Example: If a circuit has a voltage of 12 volts applied across a resistor with a resistance of 4 ohms, the current flowing through the resistor can be calculated using Ohm's law: I = V/R I = 12V / 4Ω I = 3A. Therefore, the current flowing through the resistor is 3 amperes.

Table of Vocabulary:
A conductor is a material that allows the flow of electric current through it. Conductors have a high density of free electrons that are able to move easily in response to an applied electric field.
Voltage is the force that drives electric current through a conductor.
 Knowt
Knowt