
Chapter 14: Carboxylic Acids, Esters, Amines, and Amides
14.1: Carboxylic Acids
Carboxylic Acids
These are weak acids. They have a sour or tart taste, produce hydronium ions in water, and neutralize bases.
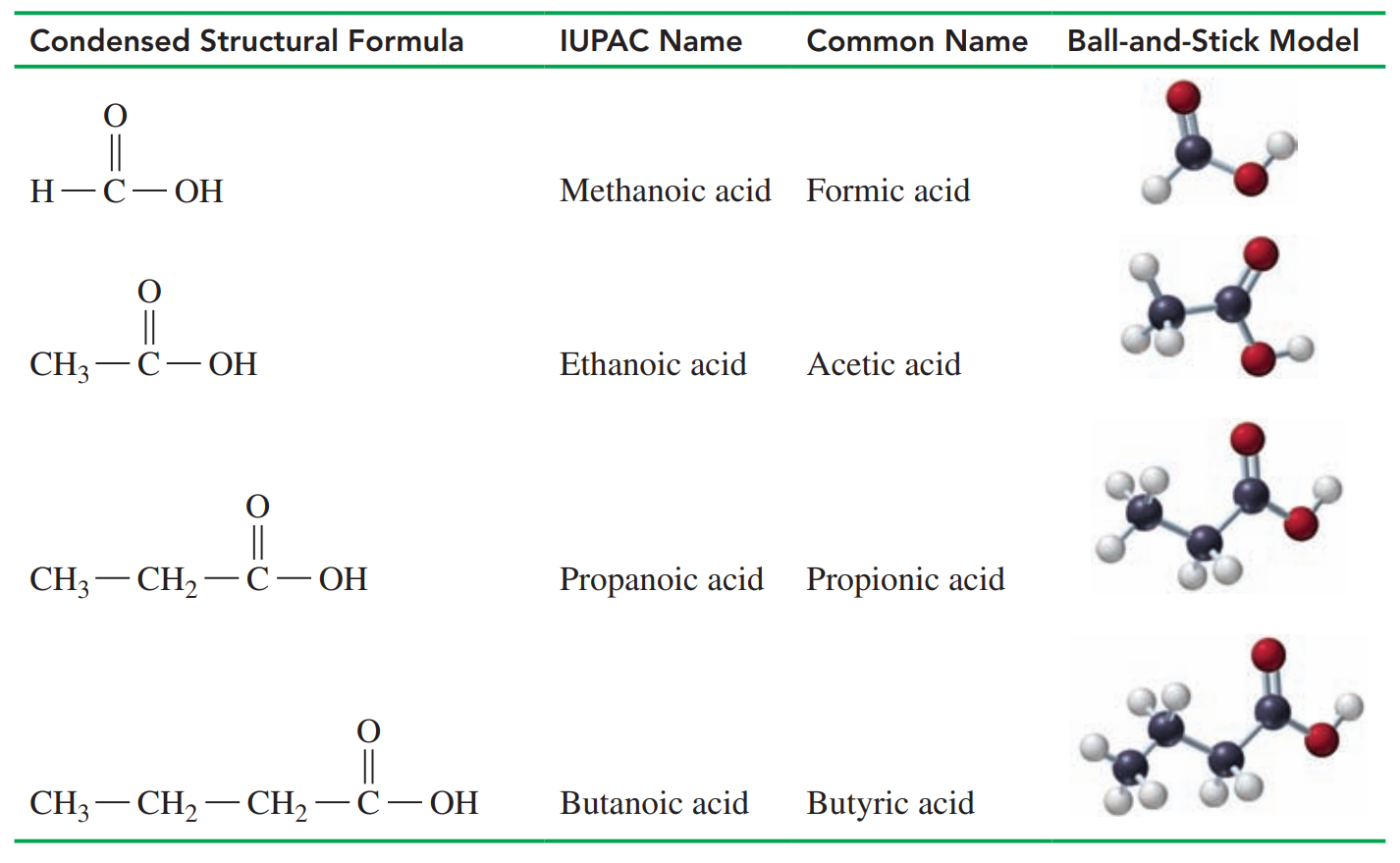
The carbon atom of a carbonyl group is attached to a hydroxyl group that forms a carboxyl group.
Common Names of Carboxylic Acids
14.2: Properties of Carboxylic Acids
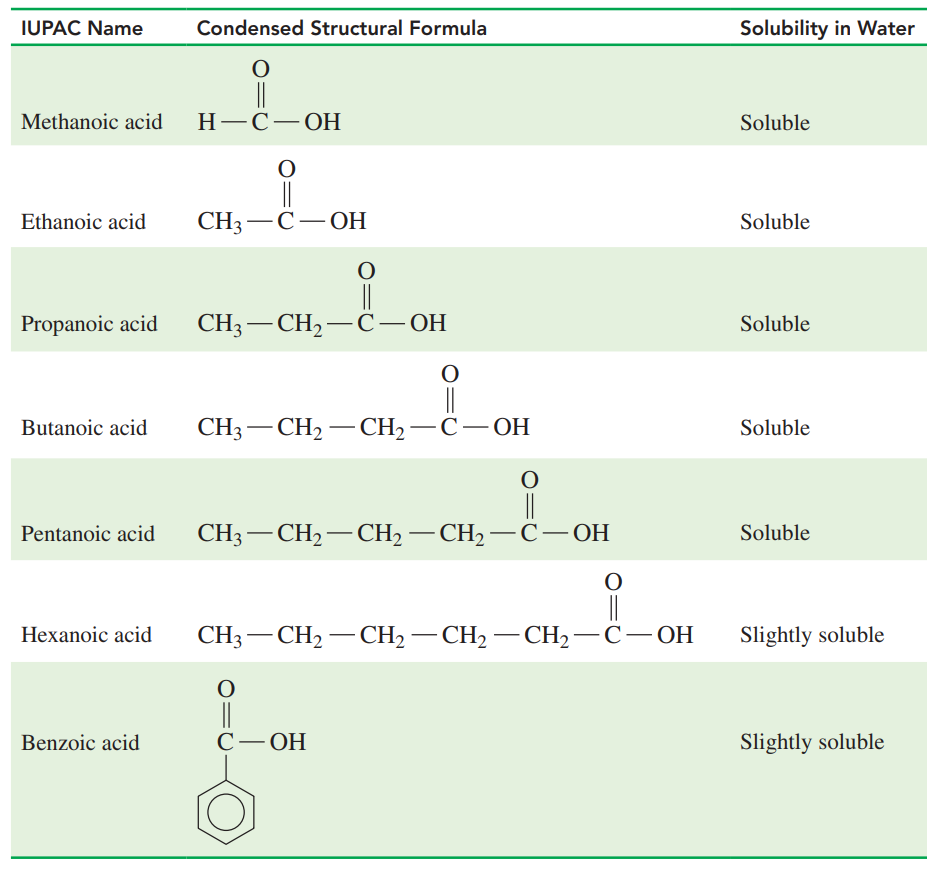
Carboxylic acids with one to five carbons are soluble in water because the carboxyl group forms hydrogen bonds with several water molecules.
When a carboxylic acid ionizes in water, a hydrogen ion is transferred to a water molecule to form a negatively charged carboxylate ion and a hydronium ion.
Because carboxylic acids are weak acids, they are completely neutralized by strong bases — the products are carboxylate salt and water.
Solubility of Selected Carboxylic Acids
14.3: Esters
A carboxylic acid reacts with an alcohol to form an ester and water.
In an ester, the —H of the carboxylic acid is replaced by an alkyl group
Esterification
An ester is produced when a carboxylic acid and an alcohol react in the presence of an acid catalyst ad heat.
The —OH group from the carboxylic acid and the —H from the alcohol are removed and combine to form water.
An excess of the alcohol is used to shift the equilibrium in the direction of the formation of the ester product.
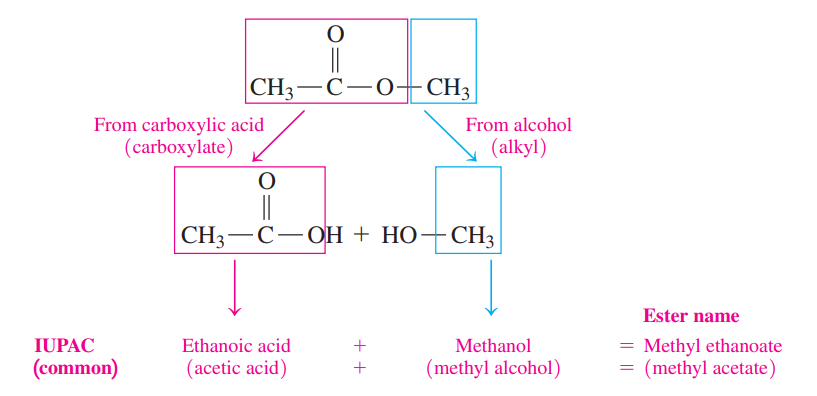
The first word indicates the alkyl part from the alcohol. The second word is the name of the carboxylate from the carboxylic acid.
The IUPAC names of esters use the IUPAC names of the acids, while the common names of esters use the common names of the acids.
14.4: Hydrolysis of Esters
Acid Hydrolysis
It occurs when esters are heated with water in the presence of a strong acid.
Water reacts with the ester to form a carboxylic acid and an alcohol.
It is the reverse of the esterification reaction.
Base Hydrolysis
It is also known as saponification, which refers to the reaction of an ester of a long-chain fatty acid with NaOH to make soap.
When an ester undergoes hydrolysis with a strong base, the products are the carboxylate salt and the corresponding alcohol.
14.5: Amines
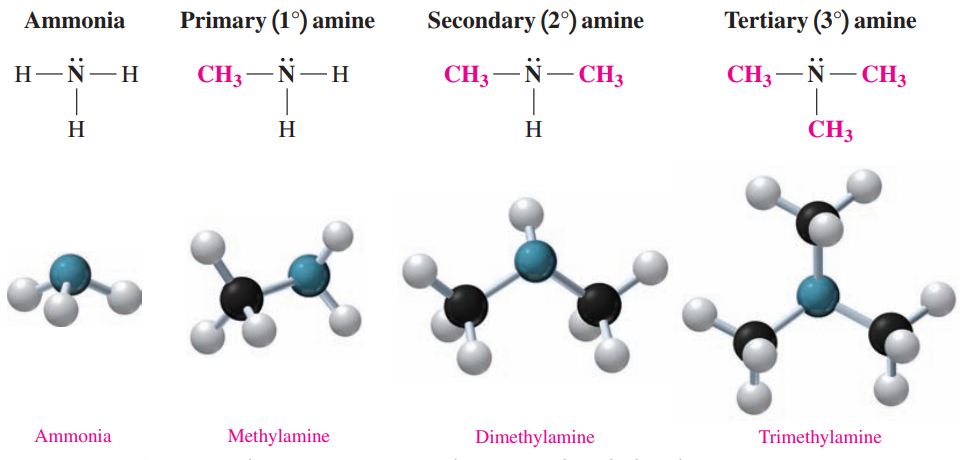
Amines: These are derivatives of ammonia in which one or more hydrogen atoms are replaced with alkyl or aromatic groups.
In methylamine, a methyl group replaces one hydrogen atom in ammonia.
The bonding of two methyl groups gives dimethylamine.
The bonding of three methyl groups in trimethylamine replaces all the hydrogen atoms in ammonia.
Amines are classified by counting the number of carbon atoms directly bonded to the nitrogen atom.
In a primary (1°) amine, the nitrogen atom is bonded to one alkyl group.
In a secondary (2°) amine, the nitrogen atom is bonded to two alkyl groups.
In a tertiary (3°) amine, the nitrogen atom is bonded to three alkyl groups.
The aromatic amines use the name aniline, which is approved by IUPAC.
Alkyl groups attached to the nitrogen of aniline are named with the prefix N- followed by the alkyl name.
Examples: Aniline; 4-Bromoaniline; N-Methylaniline
Because amines contain a polar N—H bond, they form hydrogen bonds with water.
In a primary (1°) amine, —NH2 can form more hydrogen bonds than the secondary (2°) amine.
In a tertiary (3°) amine, which has no hydrogen on the nitrogen atom, can form only hydrogen bonds with water from the N atom in the amine to the H of a water molecule.
Ammonia acts as a Brønsted–Lowry base because it accepts H+ from water to produce an ammonium ion and a hydroxide ion.
In a neutralization reaction, an amine acts as a base and reacts with an acid to form an ammonium salt.
Ammonium salts (NH4Cl)
These are ionic compounds with strong attractions between the positively charged ammonium ion and an anion, usually chloride.
These salts are solids at room temperature, odorless and soluble in water and body fluids.
Amines used as drugs are usually converted to ammonium salts.
Heterocyclic amine
A cyclic compound that contains one or more nitrogen atoms in the ring.
The rings typically consist of five or six atoms and one or more nitrogen atoms.
Pyrrolidine: A ring of four carbon atoms and a nitrogen atom, all with single bonds.
Piperidine: A six-atom heterocyclic ring with a nitrogen atom.
14.6: Amides
Amides (CO-NH): These are derivatives of carboxylic acids in which a nitrogen group replaces the hydroxyl group.
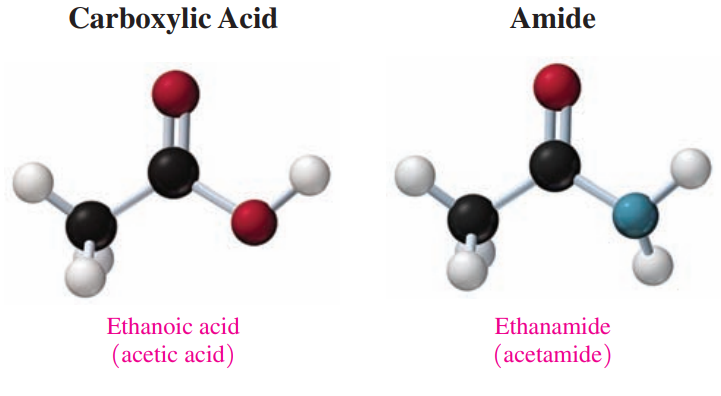
Amidation: A reaction where an amide is produced, in which a carboxylic acid reacts with ammonia or a primary or secondary amine.
In both the IUPAC and common names, amides are named by dropping the –oic acid or —ic acid from the carboxylic acid name and adding the suffix amide.
Example: N, N-Dimethylbutanamide (IUPAC); N, N-Dimethylbutyramide (Common Name)
The amides with one to five carbon atoms are soluble in water because they can hydrogen bond with water molecules.
In amides with more than five carbon atoms, the effect of hydrogen bonding is diminished as the longer carbon chain decreases the solubility of an amide in water.
Amides undergo hydrolysis when water is added to the amide bond to split the molecule.
When an acid is used, the hydrolysis products of an amide are the carboxylic acid and the ammonium salt.
In base hydrolysis, the amide produces the carboxylate salt and ammonia or an amine.
Chapter 14: Carboxylic Acids, Esters, Amines, and Amides
14.1: Carboxylic Acids
Carboxylic Acids
These are weak acids. They have a sour or tart taste, produce hydronium ions in water, and neutralize bases.
The carbon atom of a carbonyl group is attached to a hydroxyl group that forms a carboxyl group.
Common Names of Carboxylic Acids

14.2: Properties of Carboxylic Acids
Carboxylic acids with one to five carbons are soluble in water because the carboxyl group forms hydrogen bonds with several water molecules.
When a carboxylic acid ionizes in water, a hydrogen ion is transferred to a water molecule to form a negatively charged carboxylate ion and a hydronium ion.
Because carboxylic acids are weak acids, they are completely neutralized by strong bases — the products are carboxylate salt and water.
Solubility of Selected Carboxylic Acids

14.3: Esters
A carboxylic acid reacts with an alcohol to form an ester and water.
In an ester, the —H of the carboxylic acid is replaced by an alkyl group
Esterification
An ester is produced when a carboxylic acid and an alcohol react in the presence of an acid catalyst ad heat.
The —OH group from the carboxylic acid and the —H from the alcohol are removed and combine to form water.
An excess of the alcohol is used to shift the equilibrium in the direction of the formation of the ester product.
The first word indicates the alkyl part from the alcohol. The second word is the name of the carboxylate from the carboxylic acid.
The IUPAC names of esters use the IUPAC names of the acids, while the common names of esters use the common names of the acids.

14.4: Hydrolysis of Esters
Acid Hydrolysis
It occurs when esters are heated with water in the presence of a strong acid.
Water reacts with the ester to form a carboxylic acid and an alcohol.
It is the reverse of the esterification reaction.
Base Hydrolysis
It is also known as saponification, which refers to the reaction of an ester of a long-chain fatty acid with NaOH to make soap.
When an ester undergoes hydrolysis with a strong base, the products are the carboxylate salt and the corresponding alcohol.
14.5: Amines
Amines: These are derivatives of ammonia in which one or more hydrogen atoms are replaced with alkyl or aromatic groups.
In methylamine, a methyl group replaces one hydrogen atom in ammonia.
The bonding of two methyl groups gives dimethylamine.
The bonding of three methyl groups in trimethylamine replaces all the hydrogen atoms in ammonia.
Amines are classified by counting the number of carbon atoms directly bonded to the nitrogen atom.
In a primary (1°) amine, the nitrogen atom is bonded to one alkyl group.
In a secondary (2°) amine, the nitrogen atom is bonded to two alkyl groups.
In a tertiary (3°) amine, the nitrogen atom is bonded to three alkyl groups.

The aromatic amines use the name aniline, which is approved by IUPAC.
Alkyl groups attached to the nitrogen of aniline are named with the prefix N- followed by the alkyl name.
Examples: Aniline; 4-Bromoaniline; N-Methylaniline
Because amines contain a polar N—H bond, they form hydrogen bonds with water.
In a primary (1°) amine, —NH2 can form more hydrogen bonds than the secondary (2°) amine.
In a tertiary (3°) amine, which has no hydrogen on the nitrogen atom, can form only hydrogen bonds with water from the N atom in the amine to the H of a water molecule.
Ammonia acts as a Brønsted–Lowry base because it accepts H+ from water to produce an ammonium ion and a hydroxide ion.
In a neutralization reaction, an amine acts as a base and reacts with an acid to form an ammonium salt.
Ammonium salts (NH4Cl)
These are ionic compounds with strong attractions between the positively charged ammonium ion and an anion, usually chloride.
These salts are solids at room temperature, odorless and soluble in water and body fluids.
Amines used as drugs are usually converted to ammonium salts.
Heterocyclic amine
A cyclic compound that contains one or more nitrogen atoms in the ring.
The rings typically consist of five or six atoms and one or more nitrogen atoms.
Pyrrolidine: A ring of four carbon atoms and a nitrogen atom, all with single bonds.
Piperidine: A six-atom heterocyclic ring with a nitrogen atom.
14.6: Amides
Amides (CO-NH): These are derivatives of carboxylic acids in which a nitrogen group replaces the hydroxyl group.

Amidation: A reaction where an amide is produced, in which a carboxylic acid reacts with ammonia or a primary or secondary amine.
In both the IUPAC and common names, amides are named by dropping the –oic acid or —ic acid from the carboxylic acid name and adding the suffix amide.
Example: N, N-Dimethylbutanamide (IUPAC); N, N-Dimethylbutyramide (Common Name)
The amides with one to five carbon atoms are soluble in water because they can hydrogen bond with water molecules.
In amides with more than five carbon atoms, the effect of hydrogen bonding is diminished as the longer carbon chain decreases the solubility of an amide in water.
Amides undergo hydrolysis when water is added to the amide bond to split the molecule.
When an acid is used, the hydrolysis products of an amide are the carboxylic acid and the ammonium salt.
In base hydrolysis, the amide produces the carboxylate salt and ammonia or an amine.
 Knowt
Knowt
