
Chapter 2: The Chemistry of Life >
2.1 The Nature of Matter
Atoms are made up of subatomic particles which include protons (+ charge), neutrons (no charge), and electrons (- charge).
The center of an atom is called the Nucleus, which is made up of protons and neutrons, while electrons move around the nucleus.
The electrons around the nucleus are made up of shells: the first can contain 2 or fewer electrons, while the second shell can have up to 8 (This is referred to as the Valence shell, the outermost shell of electrons). 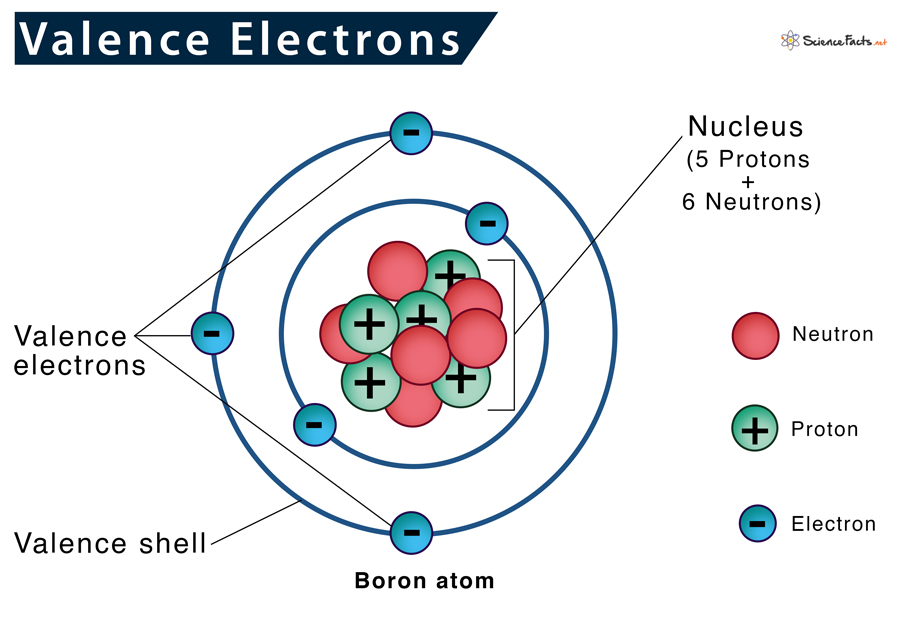
Elements
Living things are mainly composed of the 6 main elements carbon, hydrogen, nitrogen, oxygen, sulfur, and phosphorus.
Though the human body is primarily composed of oxygen, carbon, hydrogen, nitrogen, calcium, and phosphorous
Although all atoms are made up of the same few particles, they have varying properties depending on the interactions of electrons in an atom's electron shells.
Vocabulary
Atom- a basic unit of matter
Nucleus- the central core of an atom
Electron- a negatively charged particle surrounding the nucleus
Element- a pure substance; made of only one type of atom
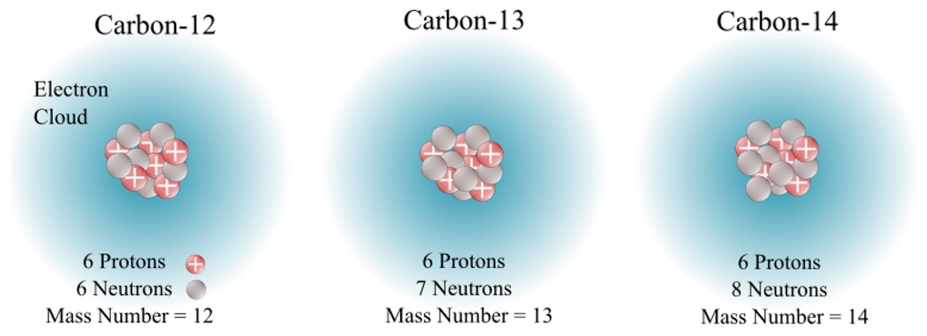
Isotope- a form of an element consisting of the same protons, but varying neutrons; different atomic mass
Compound- one type of molecule made up of more than one element
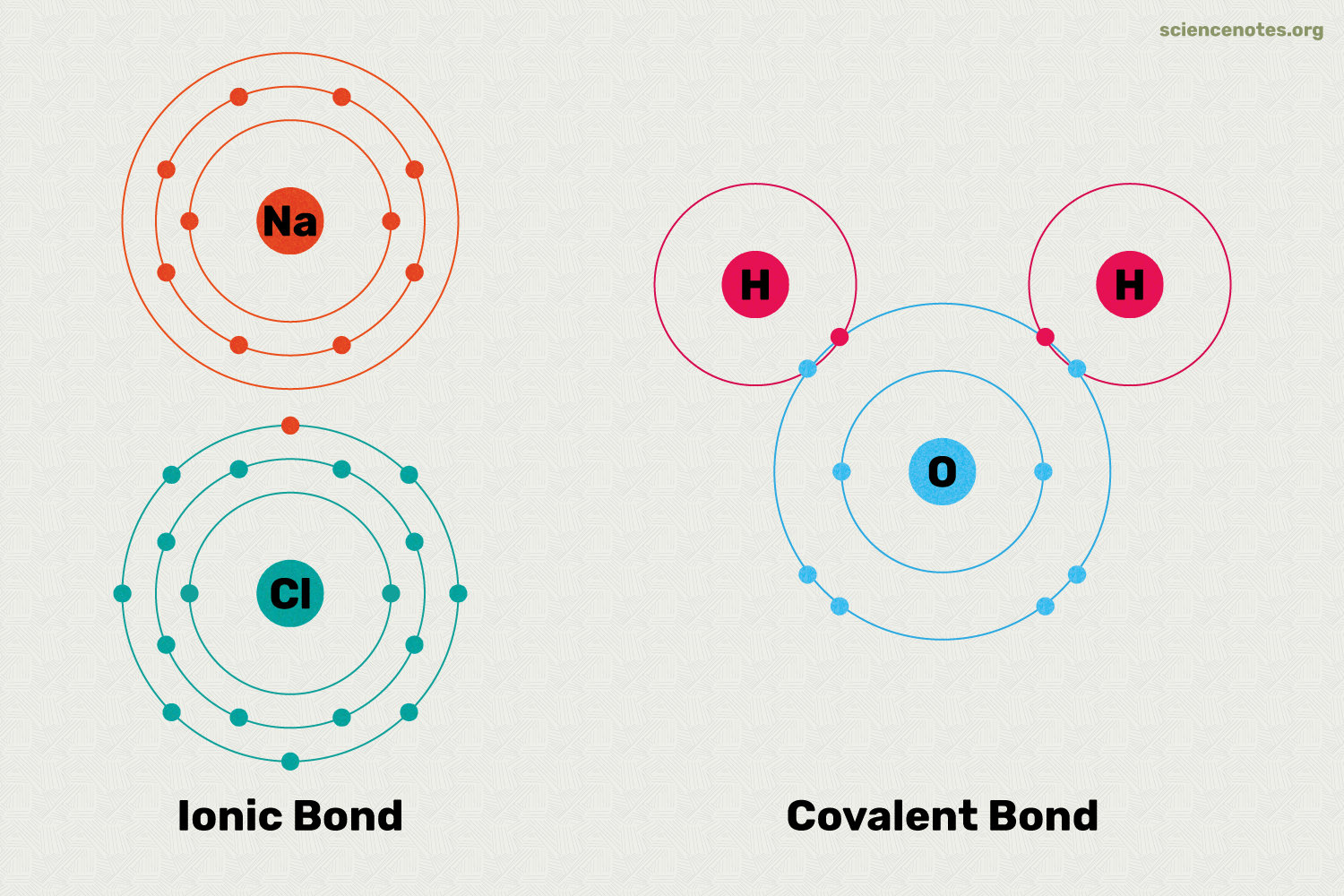
Ionic bond- a chemical bond formed from the exchange of electrons
Ion- an atom with a positive or negative charge
Covalent bond- a kind of bond between atoms formed from shared electrons
Molecule- two or more atoms joined together; the smallest particle of a substance
Van der Waals forces- A weak attraction between molecules that is dependent on distance, geckos use this to temporarily stick on walls.
Basic terminology
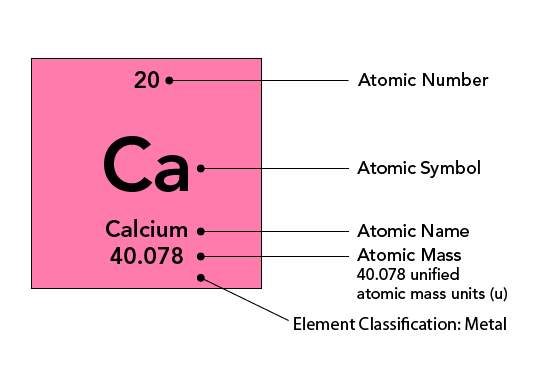
Mass number- Protons and neutrons added
Atomic mass- the weighted average of the isotopes of an element
Atomic number- the number of protons in an atom
AMU- Atomic Mass Unit
2.2 Properties of Water
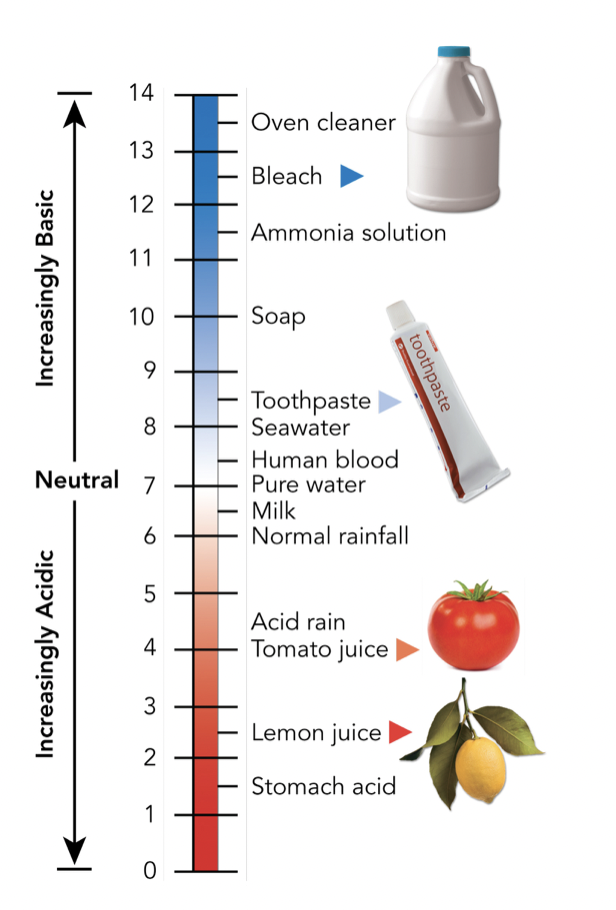
Base vs. Acidic
If you take a substance and put it in water, the substance is acidic if it causes the release of hydrogen, therefore increasing the concentration of hydrogen. If the substance decreases the concentration of hydrogen, it is a base.
Buffers are weak acids or bases which are able to react with strong acids or bases to stabilize pH changes. They are an important part of maintaining homeostasis and are able to control pH.
Cohesion vs. Adhesion
Cohesion, as the prefix co- may tell you, is the attraction between molecules of the same substance. This is the reason why water molecules are drawn together, and also how insects are able to walk on the surface of the water(cohesion produces surface tension).
Adhesion is the attraction between molecules of different substances and is the reasoning as to why the water surface in a graduated cylinder has a dip in the center of it. An effect involving adhesion is capillary action, and it is when water is able to travel up a narrow tube such as the roots of plants.
Heat capacity is the required energy needed to raise a substance's temperature. This explains why large bodies of water are able to absorb lots of heat without increasing a large amount in temperature, and why our blood doesn't overheat every time we stay in hot weather.
Solutions are mixtures that have evenly distributed components. They form when a solute is dissolved into a solvent. For example, if you mix salt into water, the salt is the solute while the water is the solvent.
Suspensions are materials that are not able to dissolve in water, but rather they separate into minuscule pieces, suspended in the liquid. For example, the cells in your blood are undissolved particles, are suspensions.
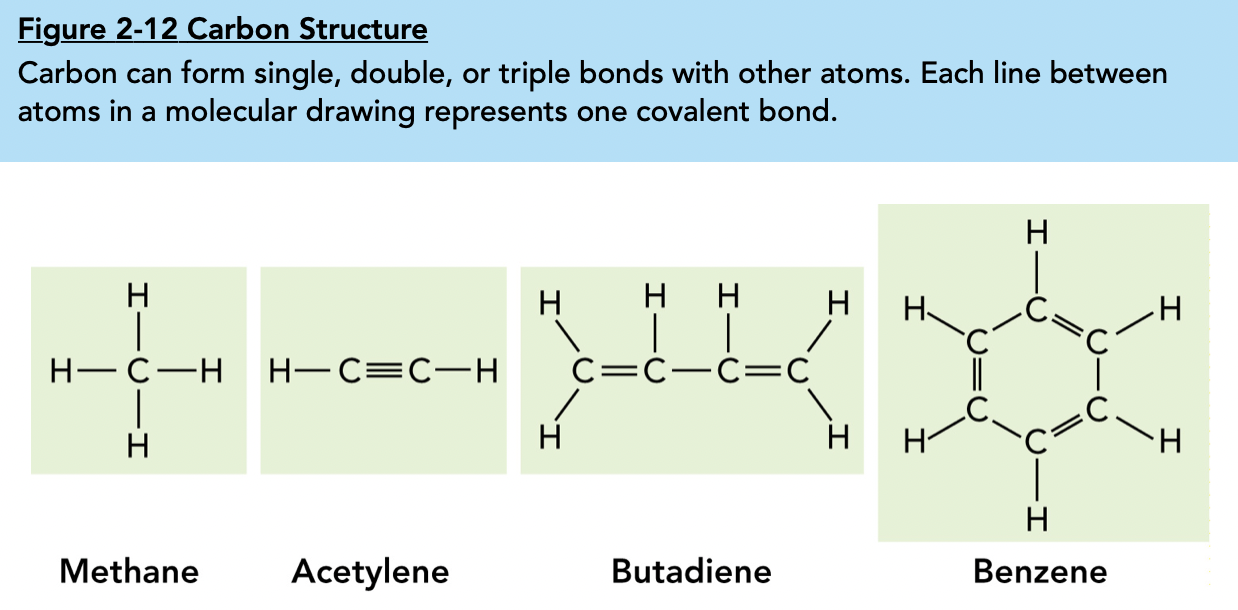
2.3 Carbon Compounds
Carbon is able to bind with various elements such as hydrogen, oxygen, phosphorus, sulfur, and nitrogen in order to form compounds.
Chapter 2: The Chemistry of Life >
2.1 The Nature of Matter
Atoms are made up of subatomic particles which include protons (+ charge), neutrons (no charge), and electrons (- charge).
The center of an atom is called the Nucleus, which is made up of protons and neutrons, while electrons move around the nucleus.
The electrons around the nucleus are made up of shells: the first can contain 2 or fewer electrons, while the second shell can have up to 8 (This is referred to as the Valence shell, the outermost shell of electrons). 
Elements
Living things are mainly composed of the 6 main elements carbon, hydrogen, nitrogen, oxygen, sulfur, and phosphorus.
Though the human body is primarily composed of oxygen, carbon, hydrogen, nitrogen, calcium, and phosphorous
Although all atoms are made up of the same few particles, they have varying properties depending on the interactions of electrons in an atom's electron shells.
Vocabulary
Atom- a basic unit of matter
Nucleus- the central core of an atom
Electron- a negatively charged particle surrounding the nucleus
Element- a pure substance; made of only one type of atom
Isotope- a form of an element consisting of the same protons, but varying neutrons; different atomic mass
Compound- one type of molecule made up of more than one element
Ionic bond- a chemical bond formed from the exchange of electrons
Ion- an atom with a positive or negative charge
Covalent bond- a kind of bond between atoms formed from shared electrons
Molecule- two or more atoms joined together; the smallest particle of a substance
Van der Waals forces- A weak attraction between molecules that is dependent on distance, geckos use this to temporarily stick on walls.


Basic terminology
Mass number- Protons and neutrons added
Atomic mass- the weighted average of the isotopes of an element
Atomic number- the number of protons in an atom
AMU- Atomic Mass Unit

2.2 Properties of Water
Base vs. Acidic
If you take a substance and put it in water, the substance is acidic if it causes the release of hydrogen, therefore increasing the concentration of hydrogen. If the substance decreases the concentration of hydrogen, it is a base.
Buffers are weak acids or bases which are able to react with strong acids or bases to stabilize pH changes. They are an important part of maintaining homeostasis and are able to control pH.

Cohesion vs. Adhesion
Cohesion, as the prefix co- may tell you, is the attraction between molecules of the same substance. This is the reason why water molecules are drawn together, and also how insects are able to walk on the surface of the water(cohesion produces surface tension).
Adhesion is the attraction between molecules of different substances and is the reasoning as to why the water surface in a graduated cylinder has a dip in the center of it. An effect involving adhesion is capillary action, and it is when water is able to travel up a narrow tube such as the roots of plants.
Heat capacity is the required energy needed to raise a substance's temperature. This explains why large bodies of water are able to absorb lots of heat without increasing a large amount in temperature, and why our blood doesn't overheat every time we stay in hot weather.
Solutions are mixtures that have evenly distributed components. They form when a solute is dissolved into a solvent. For example, if you mix salt into water, the salt is the solute while the water is the solvent.
Suspensions are materials that are not able to dissolve in water, but rather they separate into minuscule pieces, suspended in the liquid. For example, the cells in your blood are undissolved particles, are suspensions.
2.3 Carbon Compounds
Carbon is able to bind with various elements such as hydrogen, oxygen, phosphorus, sulfur, and nitrogen in order to form compounds.

 Knowt
Knowt