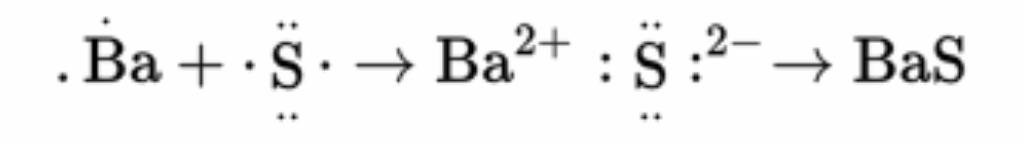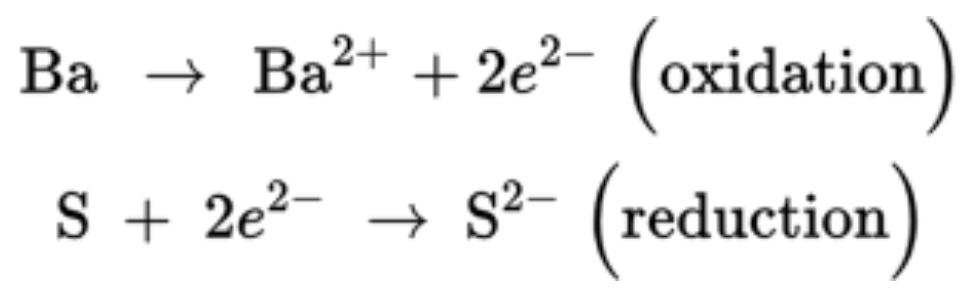Chapter 12: Oxidation-Reduction Reactions
Oxidation-reduction reactions (redox) occur when electrons are transferred from one atom to another
Oxidation is a loss of electrons
Reduction is the gain of electrons
When barium reacts with sulfur, the following reaction occurs:
The barium loses the two valence electrons and is oxidized. The sulfur gains the electrons and is reduced.
The same reaction can be written as two half-reactions to show each oxidation and reduction steps
Oxidation Numbers
Determining Oxidation Numbers
To find if an element has transferred electrons, the oxidation number (or oxidation state) is calculated
Oxidation Rule Hierarchy
The oxidation numbers of all atoms add up to the charge of the atom
Oxidation number for alkali metals is +1, alkaline earth metal charge is +2, and metals in group IIIA is +3
Hydrogen oxidation is +1, fluorine is -1
Oxygen is -2
Halogens are -1
Group VIA is -2
Rule 1 is most important and rule 6 is least important. If two rules conflict, the higher one is obeyed
Examples
For CaCl2
According to rule 2, calcium is +2. According to rule 5, each chlorine is -1. Most importantly, rule 1 indicates the oxidation state of this compound MUST be zero since the charge is zero.
Adding up the charges: (+2) + 2(-1) = 0
For KOH
Rule 2 states potassium is +1. Rule 4 states oxygen is -2. Rule 3 state hydrogen is +1. Rule 1 is followed when adding up the charges:
(+1) + (-2) + (+1) = 0
Rule 1 can be written as an equation
Total charge = (ox. no. 1)(subscript 1) + (ox. no. 2)(subscipt 2) + …
Example
For HClO4
hydrogen is +1, chlorine is -1, -2 for each oxygen. However, this disobeys rule 1 because these would add up to -8 when it needs to be zero, so rule 5 is ignored
0 charge = (ox.no. H)(1) + (ox.no. Cl)(1) + (ox.no. O)(4)
0 = (+1)(1) + (ox.no. Cl)(1) + (-2)(4)
ox.no. Cl = +7
Using Oxidation Numbers
The change in oxidation states determines how many, or if, an exchange if electrons has occurred
When permanganate reacts in an acid solution to form Mn+2, the oxidation number of manganese is +7 in the permanganate ion. This shows a redox reaction has taken place. More specifically, it has been reduced by gaining 5 electrons
Balancing Redox Reactions
Ion-electron method for balancing reactions. step 1-6 are for acid solutions (H+). Step 7 is adding only if the reaction occurs in basic solution (OH-) or H+ appears on one side and OH- on the other:
Write two half-reactions, one for ox and the other for reduc
Balance all atoms in the half-reactions except for H and O
Balance oxygen in half reaction by adding one H2O for each oxygen needed.
Balance hydrogen by adding H+
Balance the charges by adding the proper numbers of electrons. Electrons should be added to left side on one half reactions and the right side on the other half reaction
Multiply each half reaction so they each have the same number of electrons. Add the half reactinos to electrons cancel out, also common ion cancels out. Simplify coefficients
Add one OH- for each H+ ion to both sides of the equation in step 6. Combine H+ and OH- to H2O and cancel molecules on both sides and simplify if possible
For the reaction I- + IO3- → I2
I- → I2 and IO3- → I2
2I- → I2 and 2 IO3- → I2
2I- → I2 and 2 IO3- → I2 + 6 H2O
2I- → I2 and 12H+ + 2 I-3 → I2 + 6 H2O
2I- → I2 + 2e- and 10e- + 12H+ + 2 IO3- → I2 + 6 H2O
10 I- → 5 I2 + 10e- and 10e- + 12 H+ + 2 IO3- → I2 + 6 H2O
Adding equations: 10 I- + 10e- + 12 H+ + 2 IO3- → 5 I2 + 10e- + I2 + 6 H20
Canceling out: 5 I- + 6 H+ + IO3- → 3 I2 + 3H2O
Common Redox Reaction
Single Replacement (Displacement) Reactions
Single replacement is when an atom in a compound replaces another atom and produced another element and a new compound
Zn + 2 HCl → ZnCl2 + H2
Active metals can react with water in single-displacement reactions
Li, Na, K, Rb, Cs, Ca, Sr, Ba
Active metals that do not react with water but will react with acid in single-replacement
Mg, Zn, Pb, Ni, Al, Ti, Cr, Fe, Cd, Sn, Co
Inactive metals do not undergo simple single-replacement with water or acid
Ag, Pt, Au, Cu
Activity series lists metals in order of strengths to cause redox reactions
Electrochemistry
Redox reactions that would not normally occur but can occur by adding electricity in an electrolytic cell
Spontaneous redox reactions that occur without added energy create a flow of electrons in galvanic cells
Electrolysis
Electrolysis experiments would not normally occur but add two electrodes in electrically conductive sample and the voltage is adjusted until the electrons flow from electrodes
One electrode is the cathode, where the electrons are supplied
The anode electrode causes oxidation reactions to occur
Quantitative Electrochemistry
Faraday’s constant (F) is usede to convert 1 mole e- to coulombs
1 mole e- = 96,485 coulombs
moles of e- = It / F
I is coulombs per second
Time is units of seconds
Faraday’s constant
Galvanic Cells
Voltimeter readings in galvanic cells are called the standard cell voltage, E°cell, or the electromotive force, emf, or F
A thermodynamically favored reaction will give a positive voltage reading. If a reaction is not thermodynamically favored, the reaction can be reversed to make it favorable
Standard Reduction Potentials
E°cell = E°cathode - E°anode
E°cathode is standard reduction potential for the reaction occurring at the cathode and represent tendency to remove electrons from the electrode surface
E°anode is standard reduction potential for the reaction occurring at the anode and represent tendency to remove electrons from the anode
Standard Cell Voltage and Equilibrium
E°cell = (.0591 / n)(log Keq)
n is total number electrons transferred in redox reactions
combining anode and cathode quotient get equilibrium constant, Keq
Free Energy Change, ΔG°, and Standard Cell Voltages
ΔG° = -nF(E°cell)
F is faraday’s constant
Important Redox Reactions
Combustion Reaction
When organic compounds react with oxygen can cause combustion which produced carbon dioxide and water
C6H12O6 + 6 O2 → 6 CO2 + 6 H2O
Oxidation of Metals
Magnesium burns bright white in oxygen. Often in fireworks
Steel wool burns in a flame
Aluminum is considered highly combustible. However, aluminum oxide formed produces impervious coating so complete oxidation does not occur
Iron and steel react poorly. Rust requires water to occur.
Chapter 12: Oxidation-Reduction Reactions
Oxidation-reduction reactions (redox) occur when electrons are transferred from one atom to another
Oxidation is a loss of electrons
Reduction is the gain of electrons
When barium reacts with sulfur, the following reaction occurs:
The barium loses the two valence electrons and is oxidized. The sulfur gains the electrons and is reduced.
The same reaction can be written as two half-reactions to show each oxidation and reduction steps
Oxidation Numbers
Determining Oxidation Numbers
To find if an element has transferred electrons, the oxidation number (or oxidation state) is calculated
Oxidation Rule Hierarchy
The oxidation numbers of all atoms add up to the charge of the atom
Oxidation number for alkali metals is +1, alkaline earth metal charge is +2, and metals in group IIIA is +3
Hydrogen oxidation is +1, fluorine is -1
Oxygen is -2
Halogens are -1
Group VIA is -2
Rule 1 is most important and rule 6 is least important. If two rules conflict, the higher one is obeyed
Examples
For CaCl2
According to rule 2, calcium is +2. According to rule 5, each chlorine is -1. Most importantly, rule 1 indicates the oxidation state of this compound MUST be zero since the charge is zero.
Adding up the charges: (+2) + 2(-1) = 0
For KOH
Rule 2 states potassium is +1. Rule 4 states oxygen is -2. Rule 3 state hydrogen is +1. Rule 1 is followed when adding up the charges:
(+1) + (-2) + (+1) = 0
Rule 1 can be written as an equation
Total charge = (ox. no. 1)(subscript 1) + (ox. no. 2)(subscipt 2) + …
Example
For HClO4
hydrogen is +1, chlorine is -1, -2 for each oxygen. However, this disobeys rule 1 because these would add up to -8 when it needs to be zero, so rule 5 is ignored
0 charge = (ox.no. H)(1) + (ox.no. Cl)(1) + (ox.no. O)(4)
0 = (+1)(1) + (ox.no. Cl)(1) + (-2)(4)
ox.no. Cl = +7
Using Oxidation Numbers
The change in oxidation states determines how many, or if, an exchange if electrons has occurred
When permanganate reacts in an acid solution to form Mn+2, the oxidation number of manganese is +7 in the permanganate ion. This shows a redox reaction has taken place. More specifically, it has been reduced by gaining 5 electrons
Balancing Redox Reactions
Ion-electron method for balancing reactions. step 1-6 are for acid solutions (H+). Step 7 is adding only if the reaction occurs in basic solution (OH-) or H+ appears on one side and OH- on the other:
Write two half-reactions, one for ox and the other for reduc
Balance all atoms in the half-reactions except for H and O
Balance oxygen in half reaction by adding one H2O for each oxygen needed.
Balance hydrogen by adding H+
Balance the charges by adding the proper numbers of electrons. Electrons should be added to left side on one half reactions and the right side on the other half reaction
Multiply each half reaction so they each have the same number of electrons. Add the half reactinos to electrons cancel out, also common ion cancels out. Simplify coefficients
Add one OH- for each H+ ion to both sides of the equation in step 6. Combine H+ and OH- to H2O and cancel molecules on both sides and simplify if possible
For the reaction I- + IO3- → I2
I- → I2 and IO3- → I2
2I- → I2 and 2 IO3- → I2
2I- → I2 and 2 IO3- → I2 + 6 H2O
2I- → I2 and 12H+ + 2 I-3 → I2 + 6 H2O
2I- → I2 + 2e- and 10e- + 12H+ + 2 IO3- → I2 + 6 H2O
10 I- → 5 I2 + 10e- and 10e- + 12 H+ + 2 IO3- → I2 + 6 H2O
Adding equations: 10 I- + 10e- + 12 H+ + 2 IO3- → 5 I2 + 10e- + I2 + 6 H20
Canceling out: 5 I- + 6 H+ + IO3- → 3 I2 + 3H2O
Common Redox Reaction
Single Replacement (Displacement) Reactions
Single replacement is when an atom in a compound replaces another atom and produced another element and a new compound
Zn + 2 HCl → ZnCl2 + H2
Active metals can react with water in single-displacement reactions
Li, Na, K, Rb, Cs, Ca, Sr, Ba
Active metals that do not react with water but will react with acid in single-replacement
Mg, Zn, Pb, Ni, Al, Ti, Cr, Fe, Cd, Sn, Co
Inactive metals do not undergo simple single-replacement with water or acid
Ag, Pt, Au, Cu
Activity series lists metals in order of strengths to cause redox reactions
Electrochemistry
Redox reactions that would not normally occur but can occur by adding electricity in an electrolytic cell
Spontaneous redox reactions that occur without added energy create a flow of electrons in galvanic cells
Electrolysis
Electrolysis experiments would not normally occur but add two electrodes in electrically conductive sample and the voltage is adjusted until the electrons flow from electrodes
One electrode is the cathode, where the electrons are supplied
The anode electrode causes oxidation reactions to occur
Quantitative Electrochemistry
Faraday’s constant (F) is usede to convert 1 mole e- to coulombs
1 mole e- = 96,485 coulombs
moles of e- = It / F
I is coulombs per second
Time is units of seconds
Faraday’s constant
Galvanic Cells
Voltimeter readings in galvanic cells are called the standard cell voltage, E°cell, or the electromotive force, emf, or F
A thermodynamically favored reaction will give a positive voltage reading. If a reaction is not thermodynamically favored, the reaction can be reversed to make it favorable
Standard Reduction Potentials
E°cell = E°cathode - E°anode
E°cathode is standard reduction potential for the reaction occurring at the cathode and represent tendency to remove electrons from the electrode surface
E°anode is standard reduction potential for the reaction occurring at the anode and represent tendency to remove electrons from the anode
Standard Cell Voltage and Equilibrium
E°cell = (.0591 / n)(log Keq)
n is total number electrons transferred in redox reactions
combining anode and cathode quotient get equilibrium constant, Keq
Free Energy Change, ΔG°, and Standard Cell Voltages
ΔG° = -nF(E°cell)
F is faraday’s constant
Important Redox Reactions
Combustion Reaction
When organic compounds react with oxygen can cause combustion which produced carbon dioxide and water
C6H12O6 + 6 O2 → 6 CO2 + 6 H2O
Oxidation of Metals
Magnesium burns bright white in oxygen. Often in fireworks
Steel wool burns in a flame
Aluminum is considered highly combustible. However, aluminum oxide formed produces impervious coating so complete oxidation does not occur
Iron and steel react poorly. Rust requires water to occur.
 Knowt
Knowt