
Metals 12.1 to 12.4
12.1 Properties of metals
General Chemical Properties
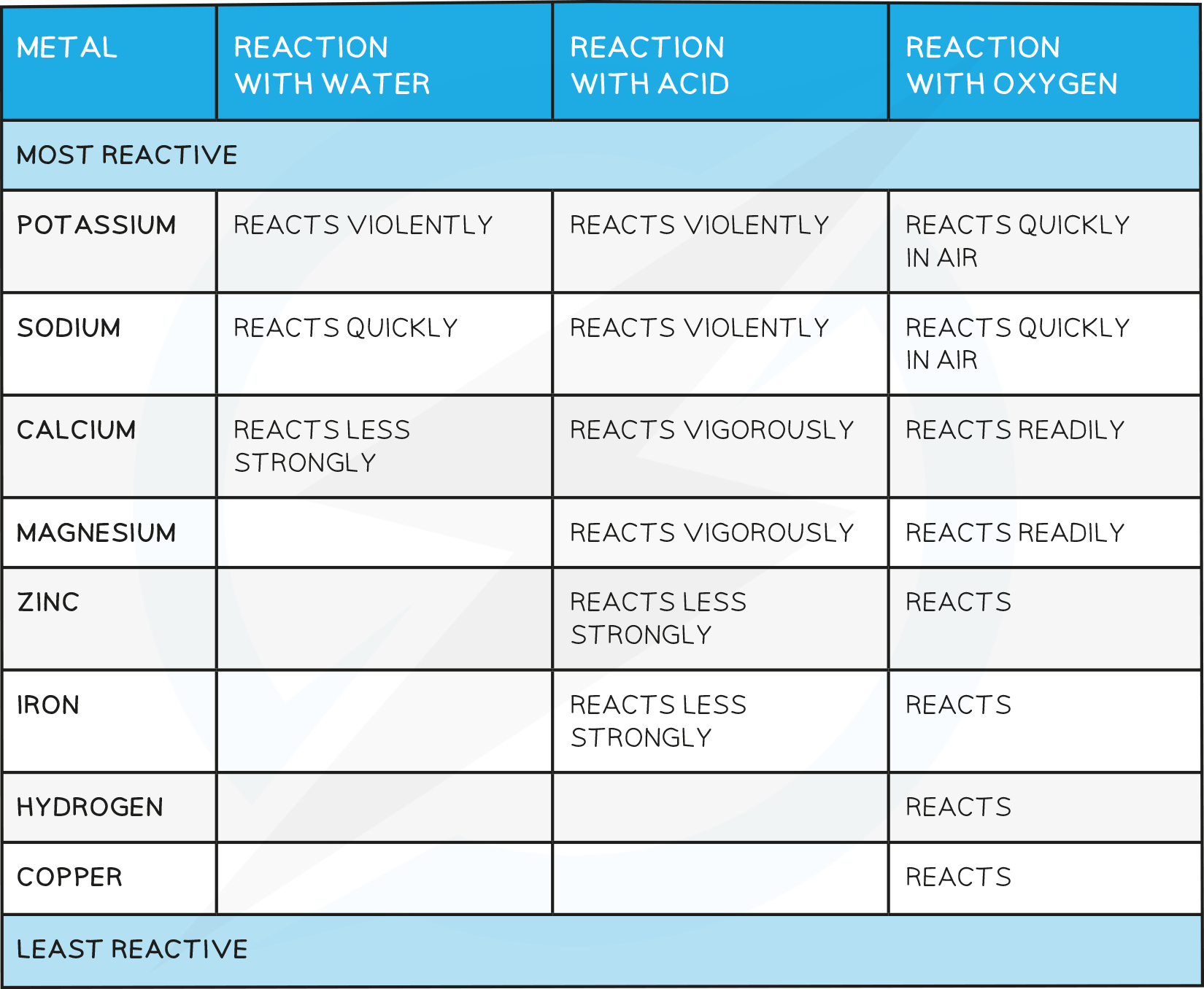
The chemistry of metals is studied by analysing their reactions with water, dilute acid and oxygen
Based on these reactions, a reactivity series of metals can be produced
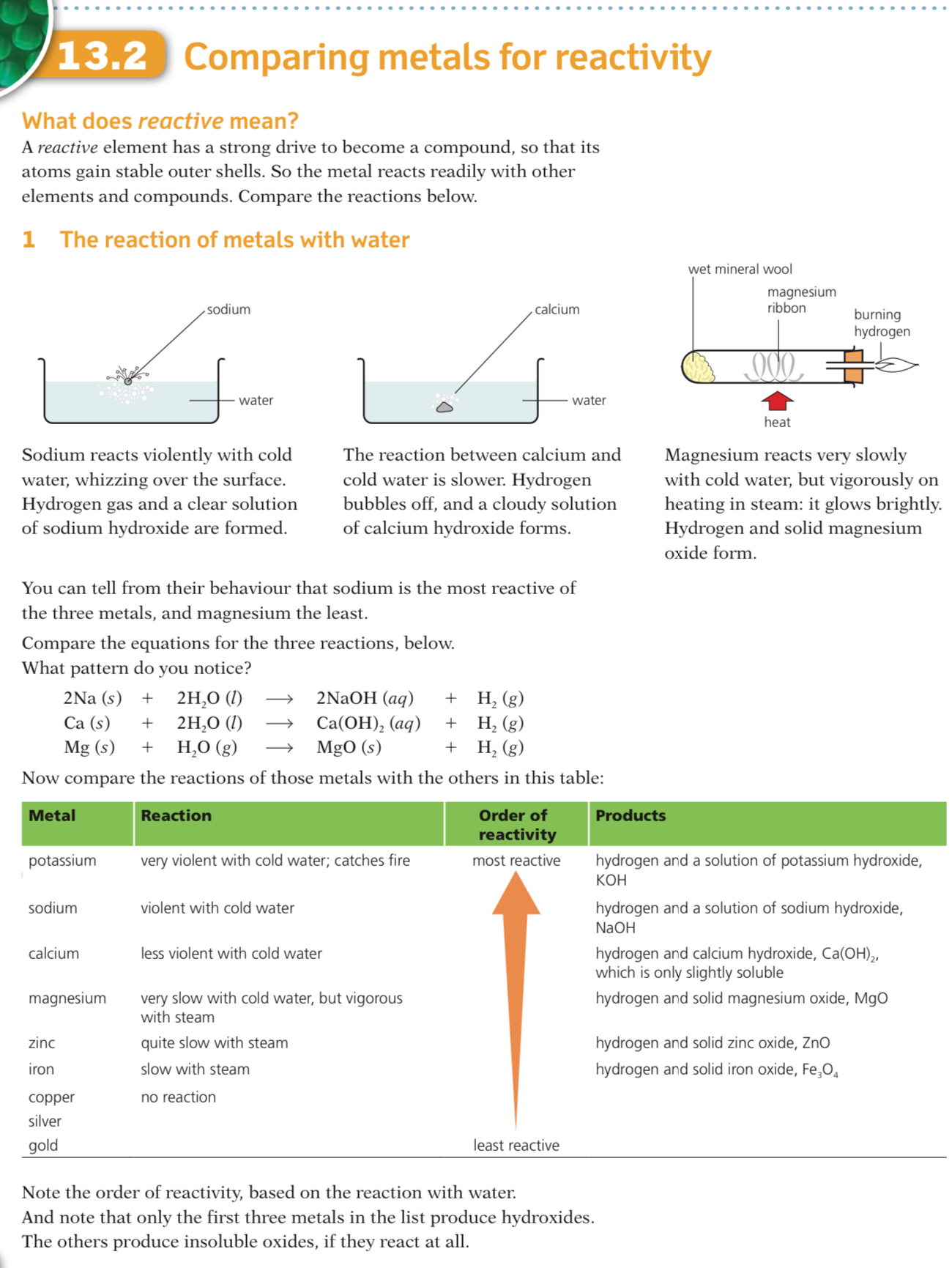
Reactivity with water
Some metals react with water, either warm or cold, or with steam
Metals that react with cold water form a metal hydroxide and hydrogen gas
metal + water → metal hydroxide + hydrogen
For example calcium:
Ca (s) + 2H2O (l) → Ca(OH)2 (aq) + H2 (g)
Metals that react with steam form metal oxide and hydrogen gas, for example zinc:
Zn (s) + H2O (l) → ZnO (s) + H2 (g)
Reactivity with acids
Most metals react with dilute acids such as HCl
When acids and metals react, the hydrogen atom in the acid is replaced by the metal atom to produce a salt and hydrogen gas, for example iron:
metal + acid → salt + hydrogen
Fe (s) + 2HCl (aq) → FeCl2 (aq) + H2 (g)
Reactivity with oxygen
Unreactive metals such as gold and copper do not react with acids
Some reactive metals such as the alkali metals react easily with oxygen
Copper and iron can also react with oxygen although much more slowly
When metals react with oxygen a metal oxide is formed, for example copper:
metal + oxygen → metal oxide
2Cu (s) + O2 (g) → 2CuO (s)
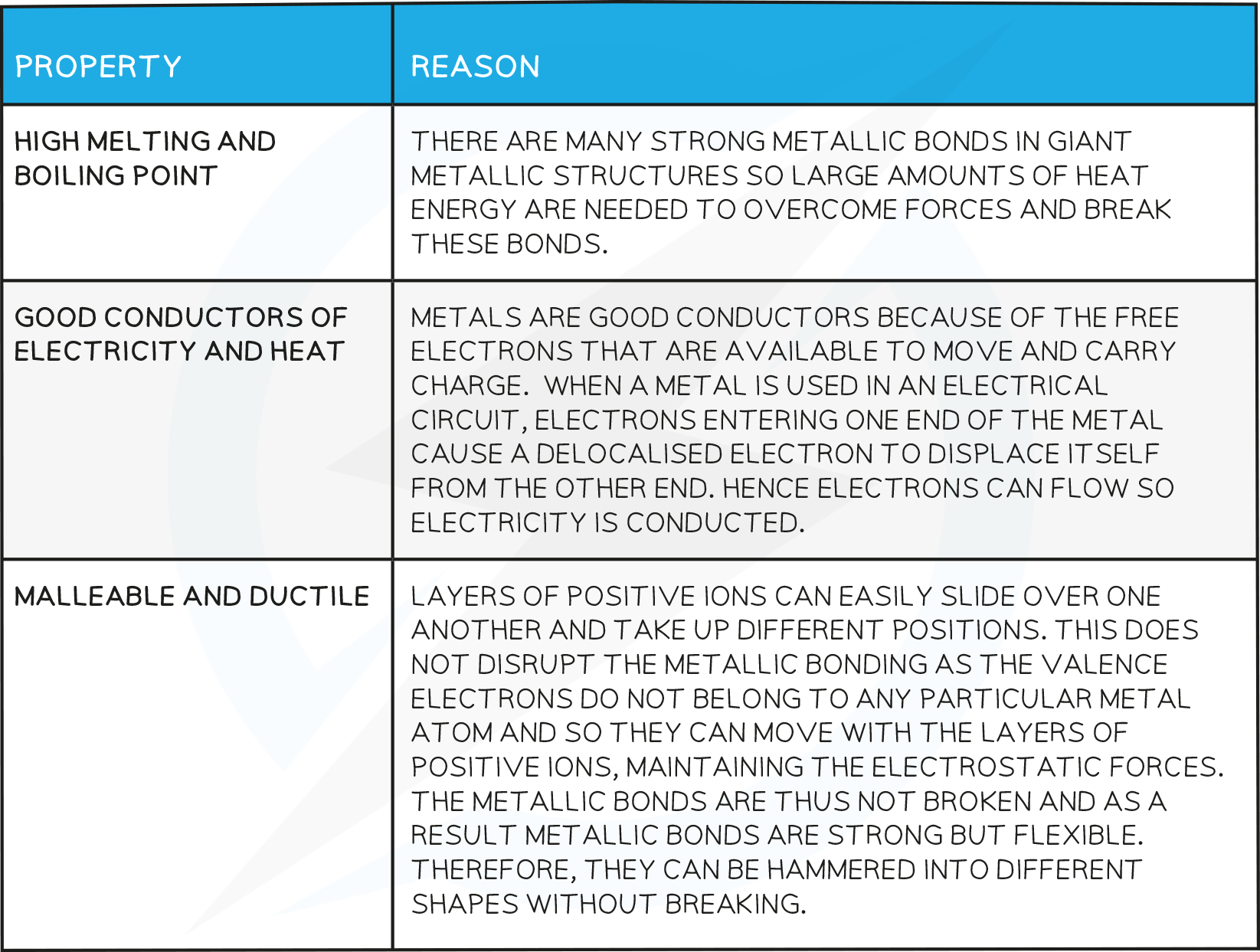
Typical physical properties of metals :
high melting points.
good conductors of electricity.
good conductors of heat.
high density.
malleable.
ductile.
Chemical Properties of Metals
The density of metals is usually high.
Metals are malleable and ductile.
Metals form an alloy with other metals or non – metals.
Some metals react with air and corrode. ...
Metals are good conductors of heat and electricity. ...
Generally, metals are in a solid state at room temperature

12.2 The Reactivity Series
Based on how metals react with water, dilute acid and oxygen, a reactivity series of metals can be produced
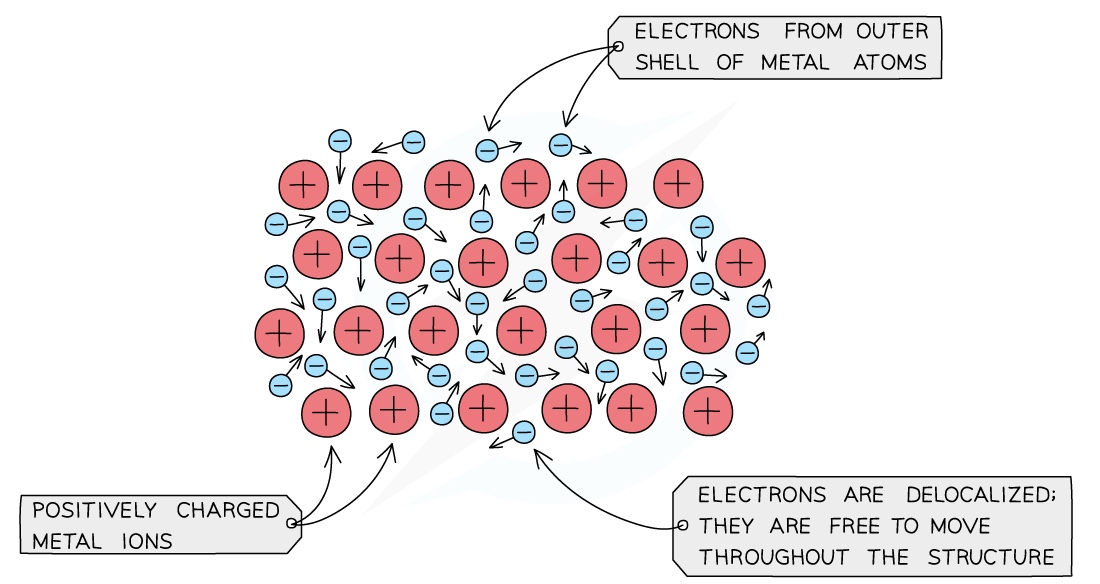
Reactivity of a metal is determined by its tendency to lose electrons and form positive ions
If a metal loses electrons easily it is more reactive than a metal that loses them less easily
The non-metal carbon is also included in the reactivity series as it is used to extract metals from their oxides
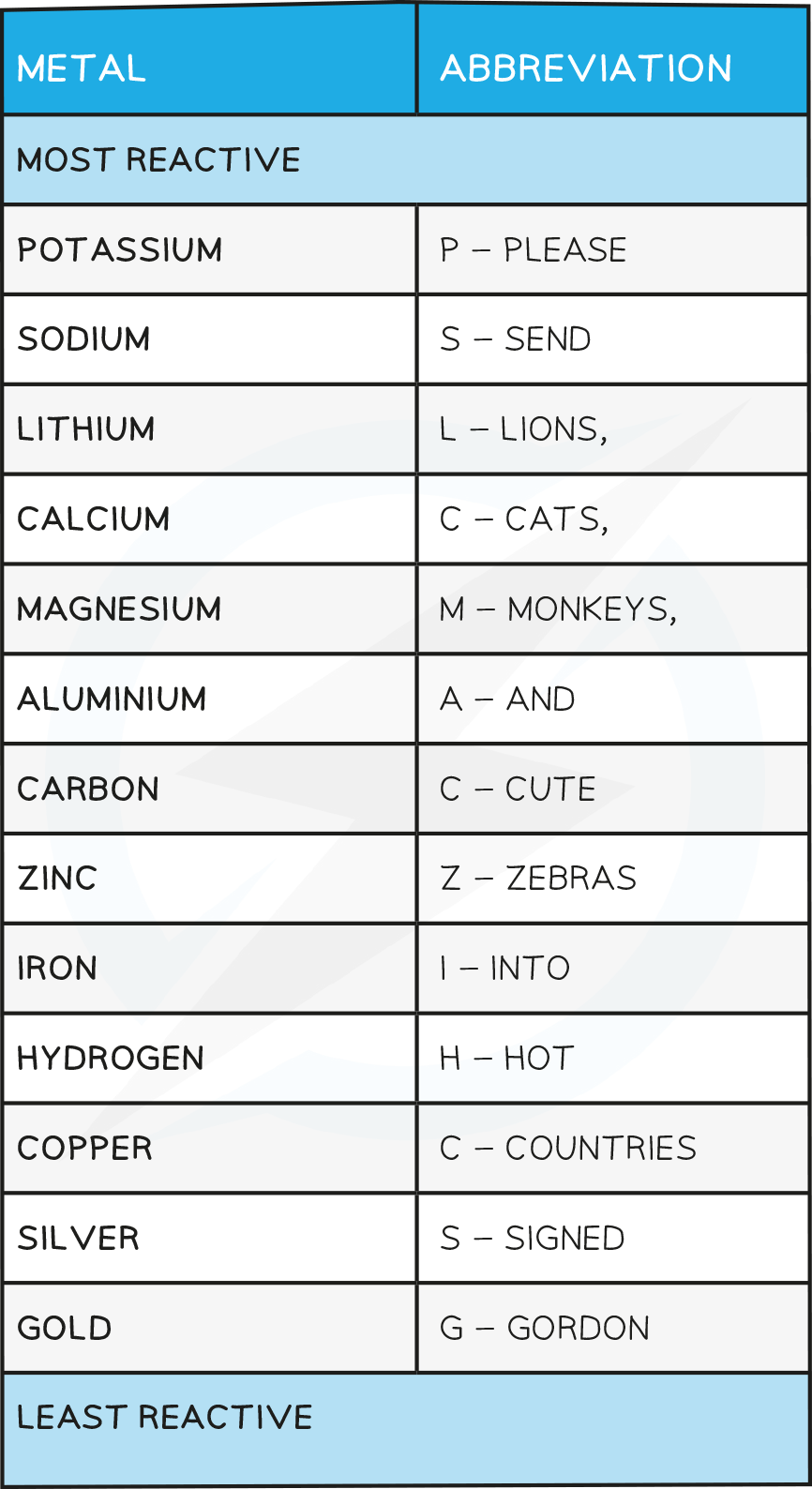
it’s important to learn The Reactivity series here’s a nemonic to help
Observations from the table above allow the following reactivity series to be deduced
The order of this reactivity series can be memorised using the following mnemonic
“Please send lions, cats, monkeys and cute zebras into hot countries signed Gordon


12.3 Extraction of Metals
Obtaining metals from there ores
The Earth’s crust contains metals and metal compounds such as gold, copper, iron oxide and aluminium oxide
Useful metals are often chemically combined with other substances forming ores
A metal ore is a rock that contains enough of the metal to make it worthwhile extracting
They have to be extracted from their ores through processes such as electrolysis, using a blast furnace or by reacting with more reactive material
In many cases the ore is an oxide of the metal, therefore the extraction of these metals is a reduction process since oxygen is being removed
Common examples of oxide ores are iron and aluminium ores which are called haematite and bauxite respectively
Unreactive metals do not have to be extracted chemically as they are often found as the uncombined element
This occurs as they do not easily react with other substances due to their chemical stability
They are known as native metals and examples include gold and platinum which can both be mined directly from the Earth’s crust
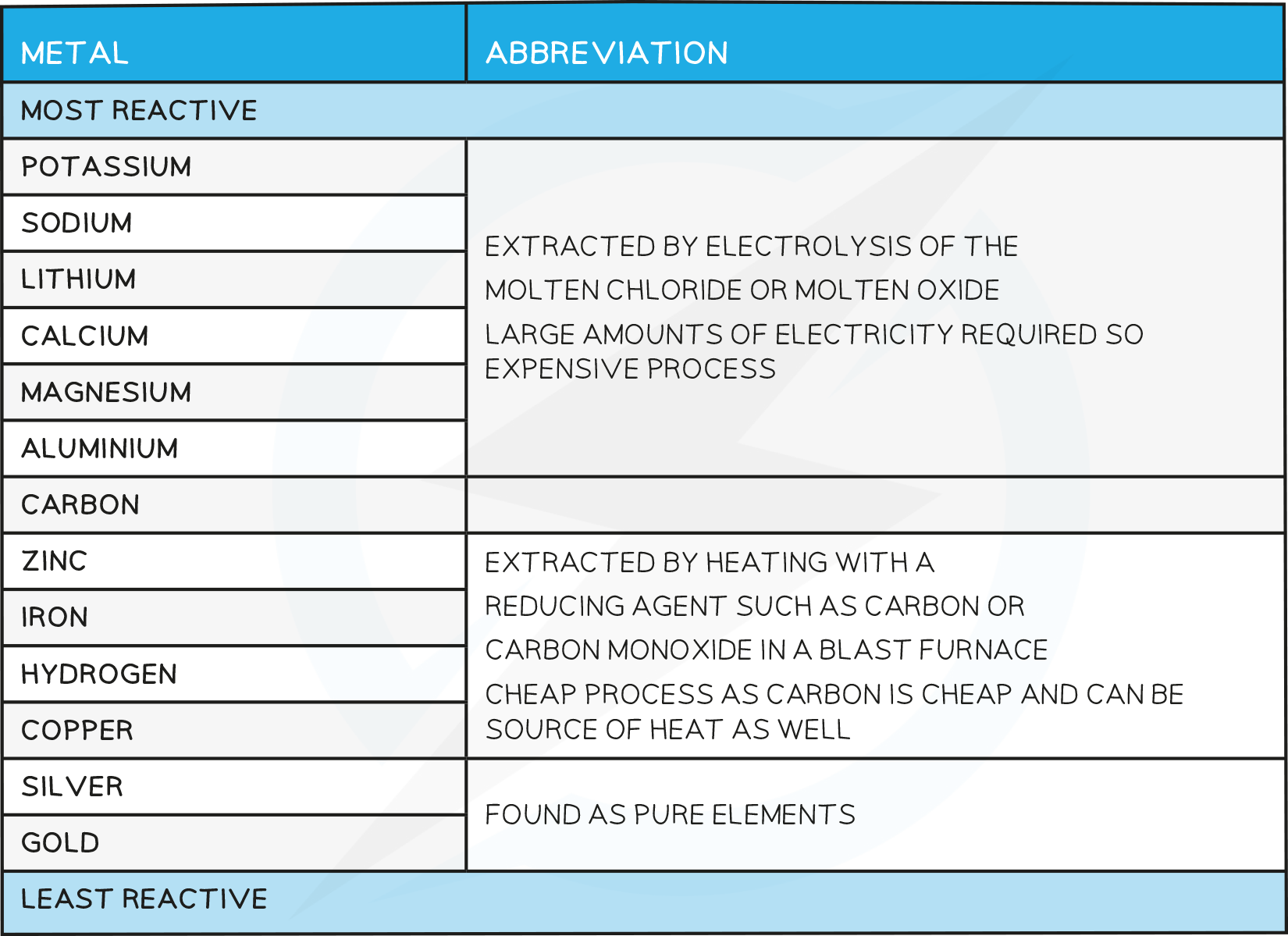
The position of the metal on the reactivity series influences the method of extraction
Those metals placed higher up on the series (above carbon) have to be extracted using electrolysis
Metals lower down on the series can be extracted by heating with carbon
Iron is extracted in a large container called a blast furnace from its ore, haematite
Modern blast furnaces produce approximately 10000 tonnes of iron per day
The process is demonstrated and explained below:
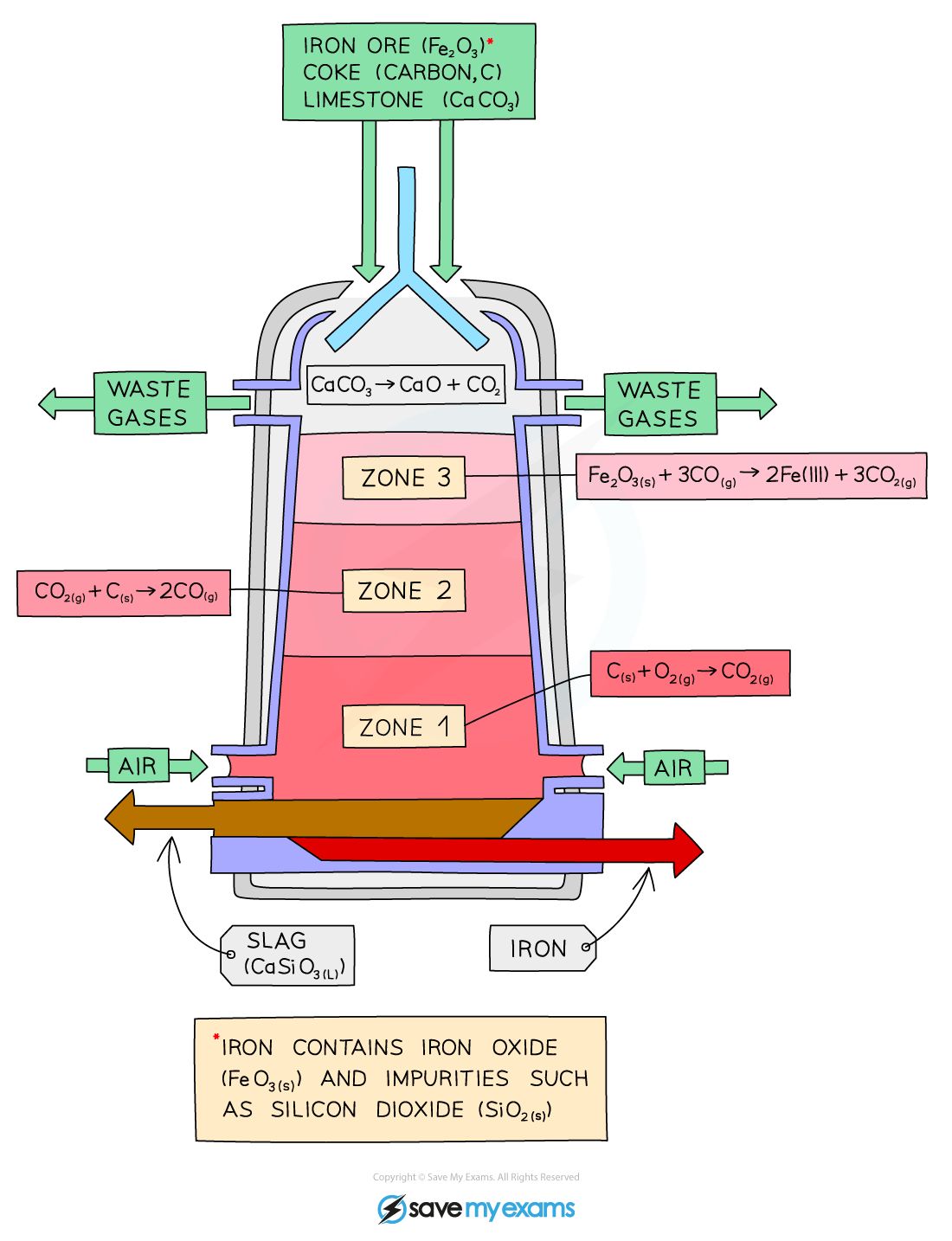
The raw materials: iron ore (haematite), coke (an impure form of carbon), and limestone are added into the top of the blast furnace
Hot air is blown in the bottom
Zone 1:
Coke burns in the hot air forming carbon dioxide
C (s) + O2 (g) → CO2 (g)
Zone 2:
At the high temperatures in the furnace, more coke reacts with carbon dioxide forming carbon monoxide
CO2 (g) + C (s) → 2CO (g)
Zone 3:
Carbon monoxide reduces the iron(III) oxide in the iron ore to form iron
This will melt and collect at the bottom of the furnace, where it is tapped off:
Fe2O3 (s) + 3CO (g) → 2Fe (I) + 3CO2 (g)
Limestone (calcium carbonate) is added to the furnace to remove impurities in the ore.
The calcium carbonate in the limestone decomposes to form calcium oxide:
CaCO3 (s) → CaO (s) + CO2 (g)
CaO (s) + SiO2 (s) → CaSiO3 (l)
The calcium oxide formed reacts with the silicon dioxide, which is an impurity in the iron ore, to form calcium silicate
This melts and collects as a molten slag floating on top of the molten Iron, which is tapped off separately:
CaO (s) + SiO2 (s) → CaSiO3 (l)
The conversion of iron into steel
Molten iron is an alloy of 96% iron, with carbon, phosphorus, silicon and sulfur impurities
It is too brittle for most uses, so most of it is converted into steel by removing some of the impurities
Not all of the carbon is removed as steel contains some carbon, the percentage of which depends on the use of the steel
The molten iron is transferred to an oxygen furnace where the conversion to steel takes place
Oxygen is added which reacts with the carbon forming carbon dioxide and carbon monoxide
These are gases so escape from the furnace
The oxygen also reacts with other impurities, silicon and phosphorus to form acidic oxides
Powdered calcium oxide is then added which is a basic oxide
The acidic silicon and phosphorus oxides react with the powdered calcium oxide and from a slag which is mainly calcium silicate:
SiO2 (l) + CaO (s) → CaSiO3 (s)
The slag floats on the surface of the molten iron and is skimmed off
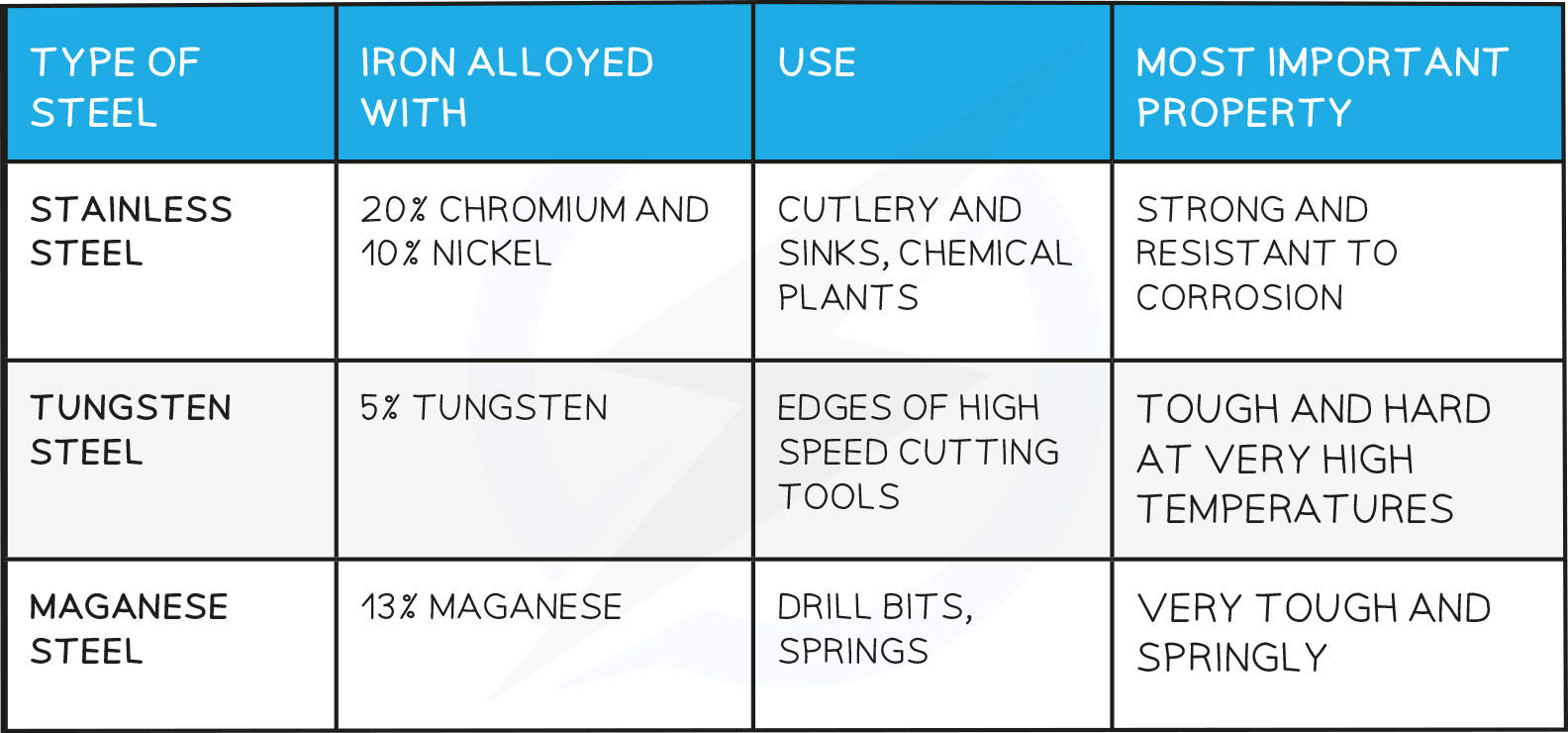
Steel Alloys
The amount of carbon removed depends on the amount of oxygen used
By carefully controlling the amount of carbon removed and subsequent addition of other metals such as chromium, manganese or nickel, the particular type of steel alloy is produced
Aluminum Extractions and Benefit of Recycling
Aluminium is a reactive metal, above carbon in the reactivity series
Its main ore, is bauxite, which is aluminium oxide
Due to aluminium's reactivity, it is extracted by electrolysis
Advantages
Raw materials are conserved (bauxite and haematite)
Reduces the amount of waste sent to landfill
Energy use is reduced, especially in the electrolysis of aluminium
Less air pollution
Disadvantages
Collection and transport of material to be recycled requires energy and fuel
Workers, vehicles and worksites need to be organised and maintained
Materials need to be sorted before they can be recycled which also requires energy and labour
Products made from recycled materials may not always be of the same quality as the original
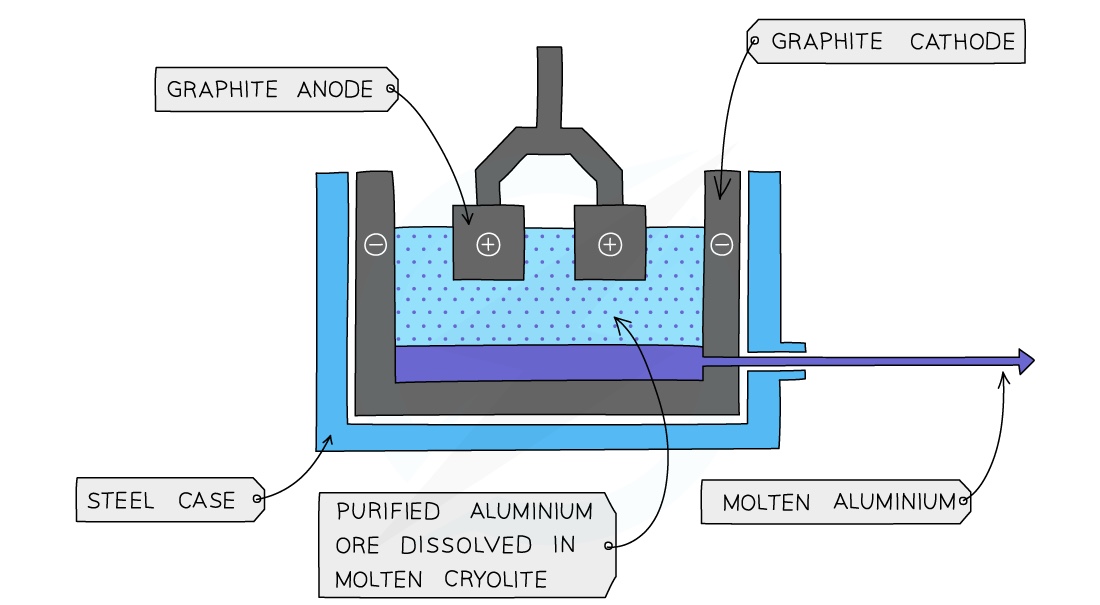
The process of Aluminum Extraction by electrolysis
Bauxite is first purified to produce aluminium oxide Al2O3
Aluminium oxide is then dissolved in molten cryolite.
This is because aluminium oxide has a melting point of over 2000°C which would use a lot of energy and be very expensive. The resulting mixture has a lower melting point without interfering with the reaction
The mixture is placed in an electrolysis cell, made from steel, lined with graphite.
The graphite lining acts as the negative electrode, with several large graphite blocks as the positive electrodes.
At the cathode:
Aluminium ions gain electrons (reduction)
Molten aluminium forms at the bottom of the cell
The molten aluminium is siphoned off from time to time and fresh aluminium oxide is added to the cell.
Al3+ 3e- → Al
At the anode:
Oxide ions lose electrons (oxidation)
Oxygen is produced at the anode:
2O2- → O2 + 4e-
The carbon in the graphite anodes reacts with the oxygen produced to produce CO2
C (s) + O2 (g) → CO2 (g)
As a result the anode wears away and has to be replaced regularly
A lot of electricity is required for this process of extraction, this is a major expense
Zinc Extraction
Zinc is a useful metal, used to galvanize iron, and used to make alloys such as brass
Zinc ore is called zinc blende, which is mainly zinc sulfide, ZnS
The zinc blende is first roasted in air to produce zinc oxide and sulfur dioxide
2ZnS (s) + O2 (g) → 2ZnO (s) + SO2 (g)
The zinc oxide is then reduced in one of two ways
Using carbon monoxide
Electrolysis
Reduction using carbon monoxide:
Carbon burns in a blast of very hot air to form carbon dioxide:
C (s) + O2 (g) → CO2 (g)
The carbon dioxide produced reacts with more coke to form carbon monoxide:
CO2 (g) + C (s) → 2CO (g)
The carbon monoxide is the reducing agent and reduces the zinc oxide to zinc:
ZnO (s) + CO (g) → Zn (s) + CO2 (g)
The mixture produced contains zinc and other impurities
It is separated by fractional distillation
Electrolysis
Zinc oxide needs to be molten but has a high melting point
Is dissolved in sulfuric acid and due to being a base, neutralises it to produce zinc sulfate
At the cathode: zinc ions gains electrons (reduction) to produce zinc
Zn2+ + 2e- → Z
Uses of zinc
Zinc is used in galvanising, the process of coating a metal such as iron or steel with a protective coating of zinc to prevent corrosion or rusting
Galvanising is an effective way of rust protection as it works even if the zinc coating becomes scratched or damaged
ZnCO3 is formed when zinc reacts with oxygen and carbon dioxide in the air and protects the iron by the barrier method
If the coating is damaged or scratched, the iron is still protected from rusting because zinc preferentially corrodes as it is higher up the reactivity series than iron
Compared to iron it loses its electrons more readily:
Zn → Zn2+ + 2e-
The iron stays protected as it accepts the electrons released by zinc, remaining in the reduced state and thus it does not undergo oxidation
The electrons donated by the zinc react with hydrogen ions in the water producing hydrogen gas
2H+ + 2e– → H2
Zinc therefore reacts with oxygen and water and corrodes instead of the iron
Zinc is also used to make an alloy called brass
Brass contains 70% copper and 30% zinc
The addition of zinc makes the alloy much harder and more corrosion resistant than copper alone
12.4 Uses of Aluminum, Copper and mild steel
Uses of Aluminium
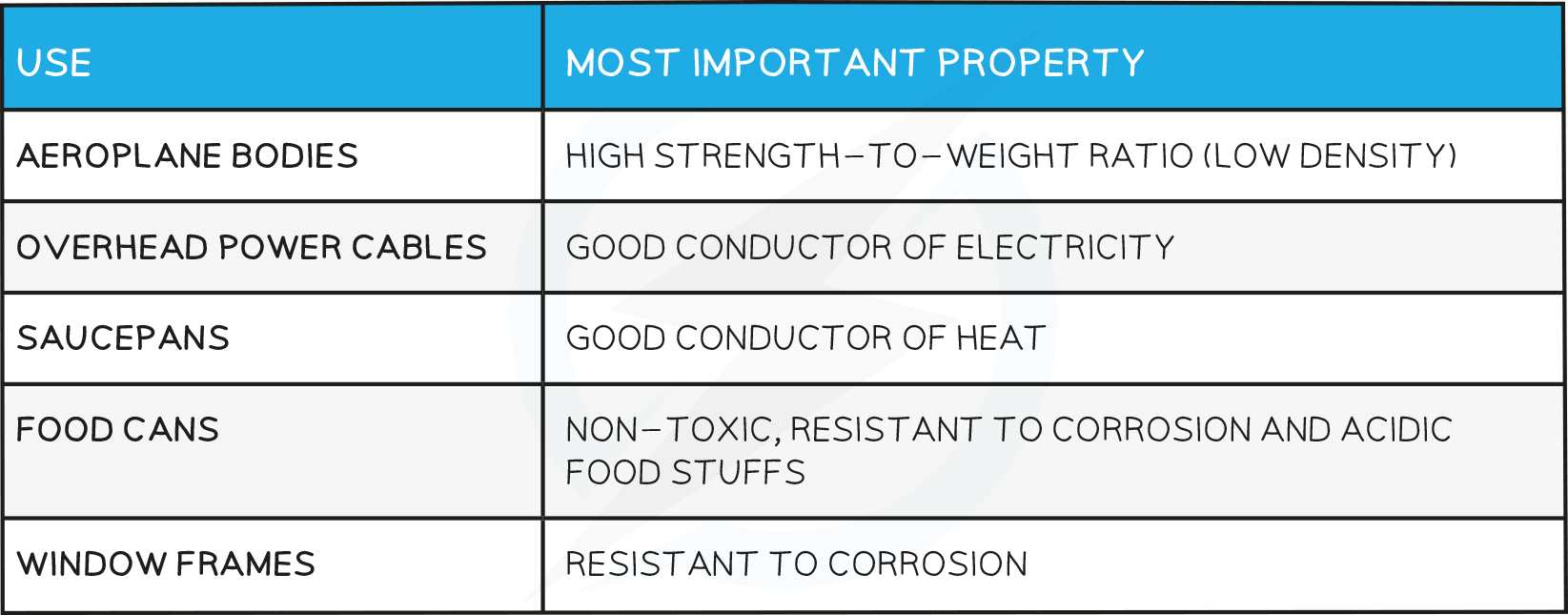
Uses of Copper
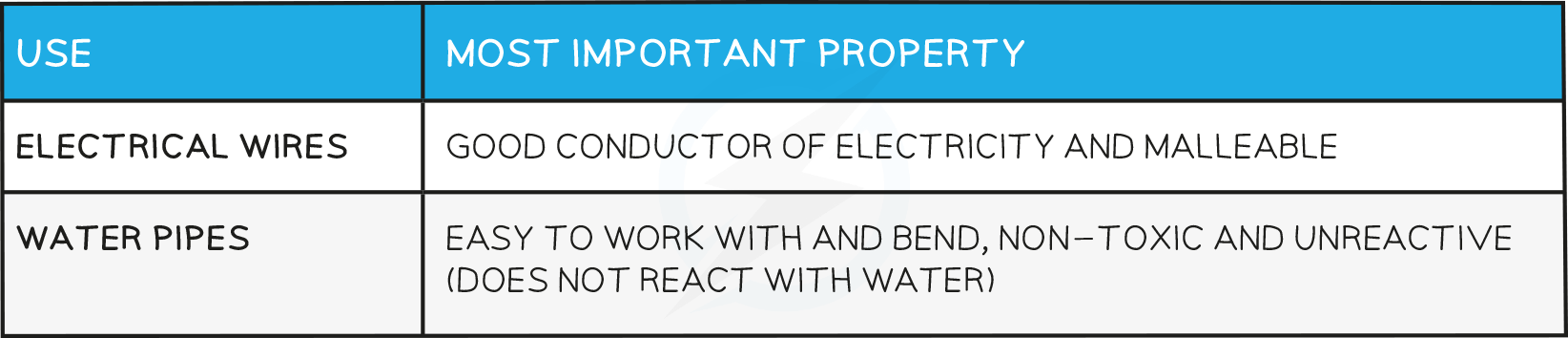
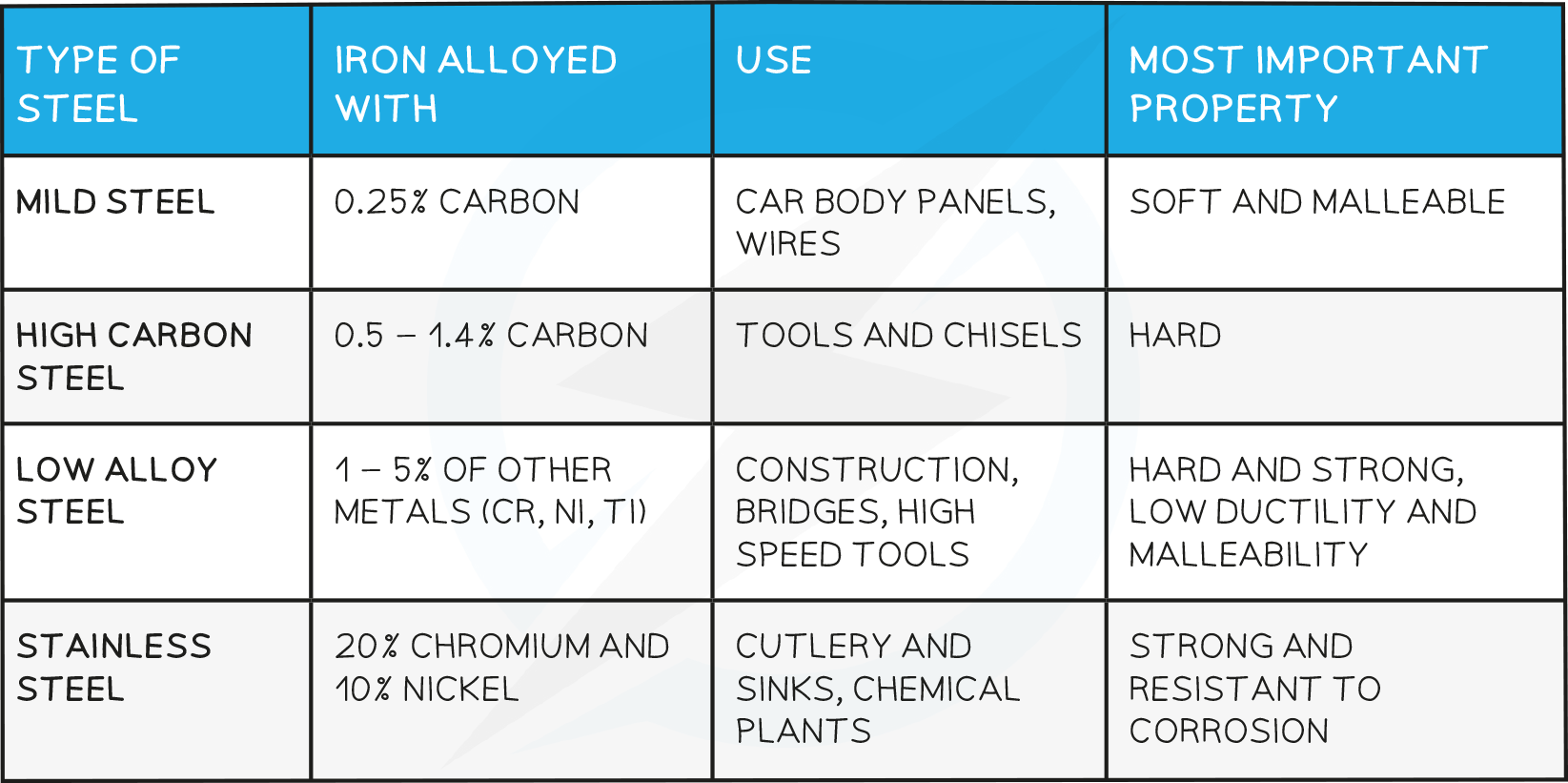
Uses of steel:
Pure iron is not useful by itself as it is too soft and rusts very easily
Adding different quantities of carbon and other metals allows the properties to be changed and therefore used in various contexts
It is important to monitor the amount of carbon that has been added to iron- too little and the iron is not strong enough but too much and the iron becomes brittle
Metals 12.1 to 12.4
12.1 Properties of metals


General Chemical Properties
The chemistry of metals is studied by analysing their reactions with water, dilute acid and oxygen
Based on these reactions, a reactivity series of metals can be produced
Reactivity with water
Some metals react with water, either warm or cold, or with steam
Metals that react with cold water form a metal hydroxide and hydrogen gas
metal + water → metal hydroxide + hydrogen
For example calcium:
Ca (s) + 2H2O (l) → Ca(OH)2 (aq) + H2 (g)
Metals that react with steam form metal oxide and hydrogen gas, for example zinc:
Zn (s) + H2O (l) → ZnO (s) + H2 (g)
Reactivity with acids
Most metals react with dilute acids such as HCl
When acids and metals react, the hydrogen atom in the acid is replaced by the metal atom to produce a salt and hydrogen gas, for example iron:
metal + acid → salt + hydrogen
Fe (s) + 2HCl (aq) → FeCl2 (aq) + H2 (g)
Reactivity with oxygen
Unreactive metals such as gold and copper do not react with acids
Some reactive metals such as the alkali metals react easily with oxygen
Copper and iron can also react with oxygen although much more slowly
When metals react with oxygen a metal oxide is formed, for example copper:
metal + oxygen → metal oxide
2Cu (s) + O2 (g) → 2CuO (s)
Typical physical properties of metals :
high melting points.
good conductors of electricity.
good conductors of heat.
high density.
malleable.
ductile.
Chemical Properties of Metals
The density of metals is usually high.
Metals are malleable and ductile.
Metals form an alloy with other metals or non – metals.
Some metals react with air and corrode. ...
Metals are good conductors of heat and electricity. ...
Generally, metals are in a solid state at room temperature

12.2 The Reactivity Series
Based on how metals react with water, dilute acid and oxygen, a reactivity series of metals can be produced
Reactivity of a metal is determined by its tendency to lose electrons and form positive ions
If a metal loses electrons easily it is more reactive than a metal that loses them less easily
The non-metal carbon is also included in the reactivity series as it is used to extract metals from their oxides


it’s important to learn The Reactivity series here’s a nemonic to help
Observations from the table above allow the following reactivity series to be deduced
The order of this reactivity series can be memorised using the following mnemonic
“Please send lions, cats, monkeys and cute zebras into hot countries signed Gordon



12.3 Extraction of Metals
Obtaining metals from there ores
The Earth’s crust contains metals and metal compounds such as gold, copper, iron oxide and aluminium oxide
Useful metals are often chemically combined with other substances forming ores
A metal ore is a rock that contains enough of the metal to make it worthwhile extracting
They have to be extracted from their ores through processes such as electrolysis, using a blast furnace or by reacting with more reactive material
In many cases the ore is an oxide of the metal, therefore the extraction of these metals is a reduction process since oxygen is being removed
Common examples of oxide ores are iron and aluminium ores which are called haematite and bauxite respectively
Unreactive metals do not have to be extracted chemically as they are often found as the uncombined element
This occurs as they do not easily react with other substances due to their chemical stability
They are known as native metals and examples include gold and platinum which can both be mined directly from the Earth’s crust
The position of the metal on the reactivity series influences the method of extraction
Those metals placed higher up on the series (above carbon) have to be extracted using electrolysis
Metals lower down on the series can be extracted by heating with carbon

Iron is extracted in a large container called a blast furnace from its ore, haematite
Modern blast furnaces produce approximately 10000 tonnes of iron per day
The process is demonstrated and explained below:

The raw materials: iron ore (haematite), coke (an impure form of carbon), and limestone are added into the top of the blast furnace
Hot air is blown in the bottom
Zone 1:
Coke burns in the hot air forming carbon dioxide
C (s) + O2 (g) → CO2 (g)
Zone 2:
At the high temperatures in the furnace, more coke reacts with carbon dioxide forming carbon monoxide
CO2 (g) + C (s) → 2CO (g)
Zone 3:
Carbon monoxide reduces the iron(III) oxide in the iron ore to form iron
This will melt and collect at the bottom of the furnace, where it is tapped off:
Fe2O3 (s) + 3CO (g) → 2Fe (I) + 3CO2 (g)
Limestone (calcium carbonate) is added to the furnace to remove impurities in the ore.
The calcium carbonate in the limestone decomposes to form calcium oxide:
CaCO3 (s) → CaO (s) + CO2 (g)
CaO (s) + SiO2 (s) → CaSiO3 (l)
The calcium oxide formed reacts with the silicon dioxide, which is an impurity in the iron ore, to form calcium silicate
This melts and collects as a molten slag floating on top of the molten Iron, which is tapped off separately:
CaO (s) + SiO2 (s) → CaSiO3 (l)
The conversion of iron into steel
Molten iron is an alloy of 96% iron, with carbon, phosphorus, silicon and sulfur impurities
It is too brittle for most uses, so most of it is converted into steel by removing some of the impurities
Not all of the carbon is removed as steel contains some carbon, the percentage of which depends on the use of the steel
The molten iron is transferred to an oxygen furnace where the conversion to steel takes place
Oxygen is added which reacts with the carbon forming carbon dioxide and carbon monoxide
These are gases so escape from the furnace
The oxygen also reacts with other impurities, silicon and phosphorus to form acidic oxides
Powdered calcium oxide is then added which is a basic oxide
The acidic silicon and phosphorus oxides react with the powdered calcium oxide and from a slag which is mainly calcium silicate:
SiO2 (l) + CaO (s) → CaSiO3 (s)
The slag floats on the surface of the molten iron and is skimmed off
Steel Alloys
The amount of carbon removed depends on the amount of oxygen used
By carefully controlling the amount of carbon removed and subsequent addition of other metals such as chromium, manganese or nickel, the particular type of steel alloy is produced

Aluminum Extractions and Benefit of Recycling
Aluminium is a reactive metal, above carbon in the reactivity series
Its main ore, is bauxite, which is aluminium oxide
Due to aluminium's reactivity, it is extracted by electrolysis

Advantages
Raw materials are conserved (bauxite and haematite)
Reduces the amount of waste sent to landfill
Energy use is reduced, especially in the electrolysis of aluminium
Less air pollution
Disadvantages
Collection and transport of material to be recycled requires energy and fuel
Workers, vehicles and worksites need to be organised and maintained
Materials need to be sorted before they can be recycled which also requires energy and labour
Products made from recycled materials may not always be of the same quality as the original
The process of Aluminum Extraction by electrolysis
Bauxite is first purified to produce aluminium oxide Al2O3
Aluminium oxide is then dissolved in molten cryolite.
This is because aluminium oxide has a melting point of over 2000°C which would use a lot of energy and be very expensive. The resulting mixture has a lower melting point without interfering with the reaction
The mixture is placed in an electrolysis cell, made from steel, lined with graphite.
The graphite lining acts as the negative electrode, with several large graphite blocks as the positive electrodes.
At the cathode:
Aluminium ions gain electrons (reduction)
Molten aluminium forms at the bottom of the cell
The molten aluminium is siphoned off from time to time and fresh aluminium oxide is added to the cell.
Al3+ 3e- → Al
At the anode:
Oxide ions lose electrons (oxidation)
Oxygen is produced at the anode:
2O2- → O2 + 4e-
The carbon in the graphite anodes reacts with the oxygen produced to produce CO2
C (s) + O2 (g) → CO2 (g)
As a result the anode wears away and has to be replaced regularly
A lot of electricity is required for this process of extraction, this is a major expense
Zinc Extraction
Zinc is a useful metal, used to galvanize iron, and used to make alloys such as brass
Zinc ore is called zinc blende, which is mainly zinc sulfide, ZnS
The zinc blende is first roasted in air to produce zinc oxide and sulfur dioxide
2ZnS (s) + O2 (g) → 2ZnO (s) + SO2 (g)
The zinc oxide is then reduced in one of two ways
Using carbon monoxide
Electrolysis
Reduction using carbon monoxide:
Carbon burns in a blast of very hot air to form carbon dioxide:
C (s) + O2 (g) → CO2 (g)
The carbon dioxide produced reacts with more coke to form carbon monoxide:
CO2 (g) + C (s) → 2CO (g)
The carbon monoxide is the reducing agent and reduces the zinc oxide to zinc:
ZnO (s) + CO (g) → Zn (s) + CO2 (g)
The mixture produced contains zinc and other impurities
It is separated by fractional distillation
Electrolysis
Zinc oxide needs to be molten but has a high melting point
Is dissolved in sulfuric acid and due to being a base, neutralises it to produce zinc sulfate
At the cathode: zinc ions gains electrons (reduction) to produce zinc
Zn2+ + 2e- → Z
Uses of zinc
Zinc is used in galvanising, the process of coating a metal such as iron or steel with a protective coating of zinc to prevent corrosion or rusting
Galvanising is an effective way of rust protection as it works even if the zinc coating becomes scratched or damaged
ZnCO3 is formed when zinc reacts with oxygen and carbon dioxide in the air and protects the iron by the barrier method
If the coating is damaged or scratched, the iron is still protected from rusting because zinc preferentially corrodes as it is higher up the reactivity series than iron
Compared to iron it loses its electrons more readily:
Zn → Zn2+ + 2e-
The iron stays protected as it accepts the electrons released by zinc, remaining in the reduced state and thus it does not undergo oxidation
The electrons donated by the zinc react with hydrogen ions in the water producing hydrogen gas
2H+ + 2e– → H2
Zinc therefore reacts with oxygen and water and corrodes instead of the iron
Zinc is also used to make an alloy called brass
Brass contains 70% copper and 30% zinc
The addition of zinc makes the alloy much harder and more corrosion resistant than copper alone
12.4 Uses of Aluminum, Copper and mild steel
Uses of Aluminium

Uses of Copper

Uses of steel:
Pure iron is not useful by itself as it is too soft and rusts very easily
Adding different quantities of carbon and other metals allows the properties to be changed and therefore used in various contexts
It is important to monitor the amount of carbon that has been added to iron- too little and the iron is not strong enough but too much and the iron becomes brittle

 Knowt
Knowt
