
Organic Chemistry
IUPAC rules: prefix (substituents) - parent (# of carbons) - suffix (what family)
Alkanes
steps for naming alkanes:
name the main chain (the longest continuous chain of carbons)
number the carbon atoms in the main chain
start at the end closest to the first substituent
identify and number the branching substituent
write the name as a single word
use hyphens to separate different prefixes and commas to separate numbers
list substituents in alphabetical order
properties of alkanes
called paraffins since they don’t react as most chemicals
will burn in a flame producing carbon dioxide, water, and heat
react with Cl2 in the presence of light to replace H’s with Cl’s
boiling points and melting points increase as size of alkane increases
forces between molecules are weak
reactions of alkanes
cracking: large alkane + hydrogen gas → smaller alkane
reforming: small alkane → larger alkane + hydrogen gas
substitution (halogenation): replace H with a halogen atom
initiated by addition of energy in the form of heat or ultraviolet light
combustion: hydrocarbon + oxygen → carbon dioxide + water
all hydrocarbons undergo combustion
cycloalkanes - alkanes that have carbon atoms forming a ring
steps for naming cycloalkanes:
count the number of carbon atoms in the ring and the number in the largest substituent chain
if the number of carbon atoms in the ring is equal or greater than the number in the substituent the compound is named as an alkyl-substituted cycloalkane
properties of cycloalkanes
melting points are affected by the shapes and way the crystals pack
don’t change uniformly
isomers: different molecules with the same molecular formula
structural isomers: different pattern of atom attachment
Alkenes and Alkynes
alkenes: contain a double bond (C=C)
one double bond = CnH2n
have straight chains and are unsaturated
polyunsaturated = many double bonds
much more reactive than alkanes
steps for naming alkenes:
find the longest continuous carbon chain containing the double bond
identity the substituents
number the chain from the end closest to the double bond
write the name in the following order:
substituents in alphabetical order
number of first carbon double bond
name of main chain
end with -ene
alkynes: contain a triple bond
one triple bond = CnH2n-2
have straight chains and are unsaturated
more reactive than alkenes
steps for naming alkynes:
find the longest continuous carbon chain that contains the triple bond
identify the substituents
number the chain from the end closest to the triple bond
write the name in the following order:
substituents in alphabetical order
number of first carbon triple bond
name of main chain
end with -yne
geometric isomerism: result of rotations around the double bond being highly restricted
different molecules of groups have different spatial orientation about the double bond
cis isomerism: groups are bonded on the same side
trans isomerism: groups are bonded on opposite sides
Aromatic Hydrocarbons
aromatic hydrocarbons: contain a ring structure with a series of alternating single and double bonds in a delocalized arrangement
in simple aromatic compounds, the benzene ring is the parent chain
if the attached benzene group is not easily named the benzene ring is the attached branch, called the phenyl group
numbering carbons on the benzene ring:
1,2 - ortho (o)
1,3 - meta (m)
1,4 - para (p)
Reactions of Hydrocarbons
substitution reaction: the hydrogen atoms in an alkane may be substituted by a halogen such as F2, Cl2, Br2
F2 reacts vigorously with alkanes
Cl2 and Br2 require heat or ultraviolet light to react
Product is a halogenated alkane of an alkyl halide
addition reaction: adding a molecule across the multiple bond (for alkenes and alkynes)
hydrogenation: adding H2
converts unsaturated molecule → saturated
alkene/alkyne + H2 → alkane
halogenation: adding X2 where X = F, Cl, Br, I
hydrohalogenation: adding HX where HX is polar
when adding a polar reagent to a double or triple bond the positive part attaches to the carbon with the most H’s
hydration: adding water
converts unsaturated hydrocarbon → alcohol
Markovnikov’s rule: in the addition of HX to alkene, the H attaches to the carbon with the most H’s, and the X attached to the end with the most alkyl substituents
aromatic compounds: have chemical reactions between those of alkanes and alkenes
undergo substitution reactions
substitution reactions with cyclohexane and bromine occur much slower and requires addition of heat
Organic Halides
organic halides: a group of compounds commonly used as refigerants (CFC’s) and non-stick coating (Teflon)
many organic halides are toxic and/or carcinogenic
properties of organic halides:
the bonds between the carbon and halogens are more polar than those between carbon and hydrogen
alkyl halides are more polar than their hydrocarbon parents
they are more soluble in polar solvents than their hydrocarbon parents and have higher boiling points
when a compound such as propane reacts with a halogen a mixture of compounds containing 1,2,3 or more halogens form
the more halogenated a compound is the more polar it is
preparing organic halides:
alkenes and alkynes readily add halogens or hydrogen halides to their double or triple bonds
Markovnikov’s rule applies when hydrogen halides are reactants
alkene/alkyne + hydrogen halide → organic halide
compounds with benzene rings have a substitution reaction
elimination reaction: a hydroxide ion is used to eliminate a hydrogen and halide ion from adjacent carbon atoms to form a double bond making an alkene
alkane + OH → alkene + water + halogen
Alcohols and Ethers
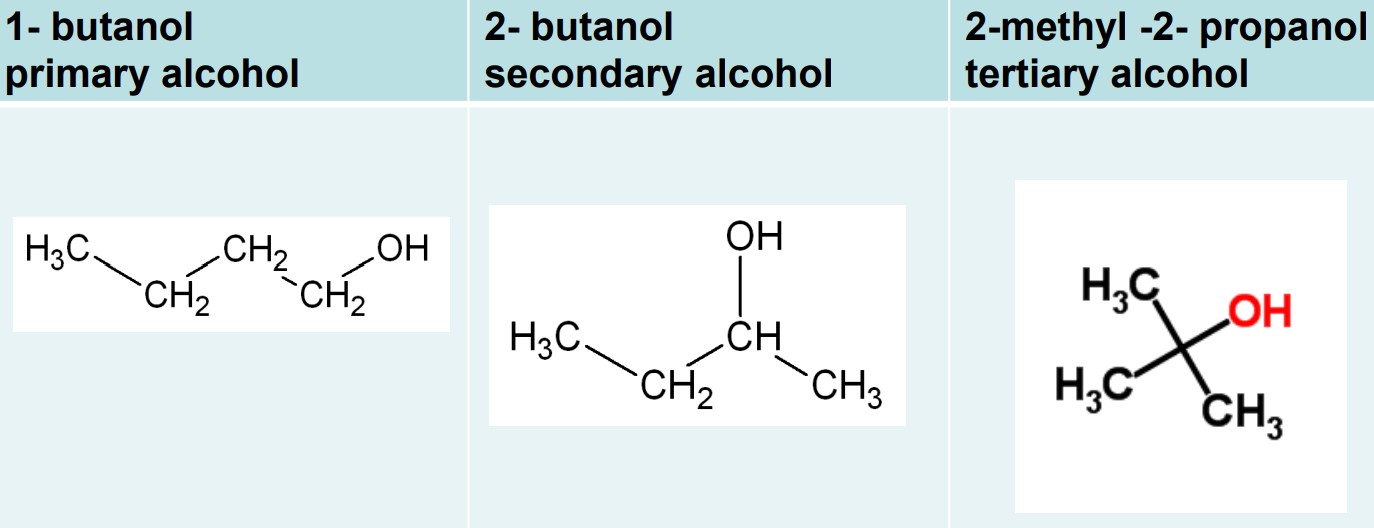
alcohol: a water molecule with one of the hydrogen atoms replaced with an alkyl group (R - O - H)
there are three classification alcohols:
primary alcohols: hydroxide is attached to an alkyl group attached to one other alkyl group
secondary alcohols: hydroxide is attached to an alkyl group attached to two other alkyl groups
tertiary alcohols: hydroxide is attached to an alkyl group attached to three other alkyl groups
cyclic alcohols: compounds containing cyclic alkanes or aromatic hydrocarbons attached to a hydroxyl group
properties of alcohols:
much higher boiling points than parent alkanes
more soluble than parent alkanes
properties are due to H-bonding among molecules
reactions involving alcohols:
hydration: reacting alkenes with water in the presence of a catalyst results in an alcohol
alkene + water → alcohol
OH is found on the second carbon due to Markovnikov’s rule
combustion: alcohols undergo complete combustion to produce carbon dioxide and water
ethers: a water molecule with both of the hydrogen atoms replaced with alkyl groups (R - O - R) or (R - O - R’)
properties of ethers:
don’t form hydrogen bonds since they lack an OH group
more polar than hydrocarbons because of the dipole arising from their C-O-C bonds
naming ethers: add oxy to the prefix of the smaller hydrocarbon group and join it to the name of the larger hydrocarbon group
preparing ethers from alcohols:
condensation: ethers are formed by the reaction of 2 alcohols and the elimination of a water molecule
alcohol + alcohol → ether + water
Aldehydes and Ketones
aldehydes: consist of an alkyl group bonded to a carbonyl group with a hydrogen atom on the end
ketones: consist of two alkyl groups attached to a central carbonyl group
naming aldehydes and ketones:
aldehyde: name ends with -al
ketones: name ends with -one
if the carbon chain has 5 or more carbon atoms a number is needed to indicate the location of the carbonyl group
properties of aldehydes and ketones:
lower boiling points and less soluble than corresponding alcohols
more soluble than corresponding alkanes
aldehydes and ketones can mix with both polar and non-polar substances
allows non-polar materials to be mixed with polar materials
preparing aldehydes and ketones from alcohols by oxidation reaction:
oxidation: reactions involve a loss of electrons
the element or compound that loses electrons is oxidized
the element or compound that gains electrons is reduced
controlled oxidation of alcohols results in aldehydes and ketones
the reactive oxygen atoms are supplied by oxidizing agents (O)
when primary alcohol is oxidized, an H atom remained on the C atom and an aldehyde is produced
primary alcohol + (O) → aldehyde + water
when secondary alcohol is oxidized, the carbonyl group that forms is attached to two alkyl groups forming a ketone
secondary alcohol + (O) → ketone + water
tertiary alcohols don’t react since there’s no hydrogen available for oxidation
hydration: hydrogen can be added to the carbonyl group in aldehydes and ketones
high temperatures and catalysts are required for this reaction
this is the reverse of the controlled oxidation of alcohols
Carboxylic Acids and Esters
carboxylic acid: characterized by the presence of carboxyl functional groups (R-COOH)
naming carboxylic acids:
the carboxyl group is made up of a hydroxyl (OH) bound to the C atom on a carbonyl group
the main chain is the longest chain containing the carboxyl group
ends with -oic acid
when naming multiple carboxyl groups the suffix -dioic is used
when more than two carboxyl groups are present, all COOH groups may be named as substituents on the parent chain
the parent chain doesn’t include the carboxylic atoms
properties of carboxylic acids:
highly polar molecules
the polarity of the carboxyl group makes carboxylic acids soluble in water
chains longer than ten carbons insoluble in water
higher boiling points than their corresponding alkanes
smaller members are soluble in water, larger carboxylic acids are relatively insoluble
conduct electricity
react with organic bases in neutralization reactions
short chain carboxylic acids are liquids at standard temperature
long chain carboxylic acids are waxy solids
preparing carboxylic acid:
when alcohol is mildy oxidized, an aldehyde is produced
if this aldehyde is oxidized further a carboxylic acid is produced
alcohol + (O) → aldehyde
aldehyde + (O) → carboxylic acid
ester: similar to carboxylic acids but th eH atom in the acid is replaced with another alkyl group (R - COO - R)
naming esters:
the name of an ester has 2 parts
first part: the name of the alkyl group used in the esterification process
second part: the name of the acid
the ending of the acid name change from -oic acid to -oate
properties of esters:
the presence of the carbonyl group make esters somewhat polar
esters are less polar than corresponding carboxylic acids because they lack an OH group capable of H bonding
they are less soluble in water with lower melting and boiling points than corresponding alcohols and carboxylic acids
smaller esters are liquid at standard temperatures while longer esters are insoluble
reactions of esters:
esterification: carboxylic acid + alcohol → ester + water
alcohol acts as an organic base and carboxylic acid acts as an acid
the ester formed is considered an organic salt
hydrolysis: reverses esterification by reacting the ester with an acid or base
ester + acid/base → acid + alcohol
a bond is broken by the addition of water resulting in two or more products
Amines and Amides
amines: ammonia with one to all of its hydrogens substituted by alkyl groups
there are three classifications of amines:
primary amine: one hydrogen is substituted with an alkyl group
prepared by reacting ammonia with an alkyl halide
ammonia + alkyl halide → primary amine + hydrogen halide
secondary amine: two hydrogens are substituted with alkyl groups
prepared by reacting a primary amine further with an alkyl halide
primary amine + alkyl halide → secondary amine + alkyl halide
tertiary amine: all three hydrogens are substituted with alkyl groups
prepared by reacting a secondary amine further with an alkyl halide
secondary amine + alkyl halide → tertiary amine + alkyl halide
naming amines:
amines are nitrogen derivatives of an alkane
amines can also be named as alkyl derivatives of ammonia
diamines: molecules with 2 amino groups
for secondary and tertiary amines include the N-prefix to show the substituted groups on the amine
properties of amines:
primary and secondary amines are very polar due to the N-H bond which allows them to H-bond with each other
tertiary amines don’t have N-H bonds and can’t H-bond
higher boiling and melting points than similar-sized ethers and alkanes
smaller amines are soluble in water
N-C and N-H bonds are more polar
amines share a lower boiling point than alcohols of similar size because N-H bonds are less polar than O-H bonds
when amines are created using alkyl halides, a mixture of primary, secondary, and tertiary amines result
can separate the different amines through boiling point
amides: hydrocarbon that contains a carbonyl group bonded to a nitrogen atom
similar to esters but the N atom replaces the O atom in the chain of an ester
they are the backbone of all protein molecules
in proteins amide bonds are called peptides
properties of amides:
amides have a polar carbonyl group and amides with a least one NH group can form strong hydrogen bonds among themselves
higher boiling points that their corresponding hydrocarbon derivatives
weak bases that are insoluble in water
low molecular weight amides are slightly soluble
can be hydrolyzed in acidic or basic conditions to produce a carboxylic acid and an amine
naming amides:
the first part of an amide’s name comes from the amine
the second part of the name comes from the acid
ends with the suffix -amide
if one or more alkyl groups is attached to the N atom the upper case N is used to clarify the location
preparing amides:
condensation: carboxylic acids react with ammonia or primary or secondary amines to produce amides
carboxylic acid + ammonia → amide + water
tertiary amines don’t undergo condensation reactions since they lack the extra H atoms needed to make water
amides can also be made with primary amines and carboxylic acids by condensation reactions
carboxylic acid + primary amine → amide + water
Synthetic Addition Polymers
polymer: made up of a group of monomers (usually 10 or more)
monomer: a hydrocarbon derived molecule
polymers may contain thousands of individual monomers, the subscript n is used to indicate the number of repeating units
polyethylene: a polymer of ethene
under certain conditions alkenes undergo addition reactions with other alkenes
the double bond in each alkene changes into a single bond freeing an unbonded electron to form a single bond with other ethene monomers
addition polymerization: consists of three stages (initiation, propagation, and termination)
an initiating molecule with an unpaired electron forms a bond with one of the carbonatoms in the double bonded monomer
this yields an unpaired electron on the other end of the monomer
this electron can then form covalen bonds with another group
the reaction continues/propigates
the chain grows until two unpaired electrons combine forming a covalent bond that links the growing chains together
properties of plastics:
plastics are chemically unreactive because chemically active unsaturated alkenes have been converted into unreactive saturated carbon chains
the strong covalent bonds make the structure very stable
most intermolecular forces are Van der waals forces but because the molecules are so large these forces are very strong
strengthening polymers with cross-linking:
alkenes with double bonds (-dienes) are monomers for several polymers
these polymers often end in -ene
cross-linking occurs between -dienes because the second double bond allows for the formation of covalent bonds between different chains of monomers
Synthetic Condensation Polymers
dimers: formed with molecules with functional groups react with other molecules
condemnation polymers: when monomers join end to end in ester or amide linkages they form polyesters and polyamides
polyesters: a polymer of carboxylic acids and alcohols
when condensation reactions are repeatedly used to join carboxylic acids and alcohols, a polyester is formed
uses a dicarboxylic acid and a diol
the acids donates the OH, the alcohol donates the H
polyamides: a polymer of carboxylic acids and amines
they are condensation polymers consisting of many amides
the monomers must contain carboxyl groups, amine groups, or one of each
Organic Chemistry
IUPAC rules: prefix (substituents) - parent (# of carbons) - suffix (what family)
Alkanes
steps for naming alkanes:
name the main chain (the longest continuous chain of carbons)
number the carbon atoms in the main chain
start at the end closest to the first substituent
identify and number the branching substituent
write the name as a single word
use hyphens to separate different prefixes and commas to separate numbers
list substituents in alphabetical order
properties of alkanes
called paraffins since they don’t react as most chemicals
will burn in a flame producing carbon dioxide, water, and heat
react with Cl2 in the presence of light to replace H’s with Cl’s
boiling points and melting points increase as size of alkane increases
forces between molecules are weak
reactions of alkanes
cracking: large alkane + hydrogen gas → smaller alkane
reforming: small alkane → larger alkane + hydrogen gas
substitution (halogenation): replace H with a halogen atom
initiated by addition of energy in the form of heat or ultraviolet light
combustion: hydrocarbon + oxygen → carbon dioxide + water
all hydrocarbons undergo combustion
cycloalkanes - alkanes that have carbon atoms forming a ring
steps for naming cycloalkanes:
count the number of carbon atoms in the ring and the number in the largest substituent chain
if the number of carbon atoms in the ring is equal or greater than the number in the substituent the compound is named as an alkyl-substituted cycloalkane
properties of cycloalkanes
melting points are affected by the shapes and way the crystals pack
don’t change uniformly
isomers: different molecules with the same molecular formula
structural isomers: different pattern of atom attachment
Alkenes and Alkynes
alkenes: contain a double bond (C=C)
one double bond = CnH2n
have straight chains and are unsaturated
polyunsaturated = many double bonds
much more reactive than alkanes
steps for naming alkenes:
find the longest continuous carbon chain containing the double bond
identity the substituents
number the chain from the end closest to the double bond
write the name in the following order:
substituents in alphabetical order
number of first carbon double bond
name of main chain
end with -ene
alkynes: contain a triple bond
one triple bond = CnH2n-2
have straight chains and are unsaturated
more reactive than alkenes
steps for naming alkynes:
find the longest continuous carbon chain that contains the triple bond
identify the substituents
number the chain from the end closest to the triple bond
write the name in the following order:
substituents in alphabetical order
number of first carbon triple bond
name of main chain
end with -yne
geometric isomerism: result of rotations around the double bond being highly restricted
different molecules of groups have different spatial orientation about the double bond
cis isomerism: groups are bonded on the same side
trans isomerism: groups are bonded on opposite sides
Aromatic Hydrocarbons
aromatic hydrocarbons: contain a ring structure with a series of alternating single and double bonds in a delocalized arrangement
in simple aromatic compounds, the benzene ring is the parent chain
if the attached benzene group is not easily named the benzene ring is the attached branch, called the phenyl group
numbering carbons on the benzene ring:
1,2 - ortho (o)
1,3 - meta (m)
1,4 - para (p)
Reactions of Hydrocarbons
substitution reaction: the hydrogen atoms in an alkane may be substituted by a halogen such as F2, Cl2, Br2
F2 reacts vigorously with alkanes
Cl2 and Br2 require heat or ultraviolet light to react
Product is a halogenated alkane of an alkyl halide
addition reaction: adding a molecule across the multiple bond (for alkenes and alkynes)
hydrogenation: adding H2
converts unsaturated molecule → saturated
alkene/alkyne + H2 → alkane
halogenation: adding X2 where X = F, Cl, Br, I
hydrohalogenation: adding HX where HX is polar
when adding a polar reagent to a double or triple bond the positive part attaches to the carbon with the most H’s
hydration: adding water
converts unsaturated hydrocarbon → alcohol
Markovnikov’s rule: in the addition of HX to alkene, the H attaches to the carbon with the most H’s, and the X attached to the end with the most alkyl substituents
aromatic compounds: have chemical reactions between those of alkanes and alkenes
undergo substitution reactions
substitution reactions with cyclohexane and bromine occur much slower and requires addition of heat
Organic Halides
organic halides: a group of compounds commonly used as refigerants (CFC’s) and non-stick coating (Teflon)
many organic halides are toxic and/or carcinogenic
properties of organic halides:
the bonds between the carbon and halogens are more polar than those between carbon and hydrogen
alkyl halides are more polar than their hydrocarbon parents
they are more soluble in polar solvents than their hydrocarbon parents and have higher boiling points
when a compound such as propane reacts with a halogen a mixture of compounds containing 1,2,3 or more halogens form
the more halogenated a compound is the more polar it is
preparing organic halides:
alkenes and alkynes readily add halogens or hydrogen halides to their double or triple bonds
Markovnikov’s rule applies when hydrogen halides are reactants
alkene/alkyne + hydrogen halide → organic halide
compounds with benzene rings have a substitution reaction
elimination reaction: a hydroxide ion is used to eliminate a hydrogen and halide ion from adjacent carbon atoms to form a double bond making an alkene
alkane + OH → alkene + water + halogen
Alcohols and Ethers
alcohol: a water molecule with one of the hydrogen atoms replaced with an alkyl group (R - O - H)
there are three classification alcohols:
primary alcohols: hydroxide is attached to an alkyl group attached to one other alkyl group
secondary alcohols: hydroxide is attached to an alkyl group attached to two other alkyl groups
tertiary alcohols: hydroxide is attached to an alkyl group attached to three other alkyl groups

cyclic alcohols: compounds containing cyclic alkanes or aromatic hydrocarbons attached to a hydroxyl group
properties of alcohols:
much higher boiling points than parent alkanes
more soluble than parent alkanes
properties are due to H-bonding among molecules
reactions involving alcohols:
hydration: reacting alkenes with water in the presence of a catalyst results in an alcohol
alkene + water → alcohol
OH is found on the second carbon due to Markovnikov’s rule
combustion: alcohols undergo complete combustion to produce carbon dioxide and water
ethers: a water molecule with both of the hydrogen atoms replaced with alkyl groups (R - O - R) or (R - O - R’)
properties of ethers:
don’t form hydrogen bonds since they lack an OH group
more polar than hydrocarbons because of the dipole arising from their C-O-C bonds
naming ethers: add oxy to the prefix of the smaller hydrocarbon group and join it to the name of the larger hydrocarbon group
preparing ethers from alcohols:
condensation: ethers are formed by the reaction of 2 alcohols and the elimination of a water molecule
alcohol + alcohol → ether + water
Aldehydes and Ketones
aldehydes: consist of an alkyl group bonded to a carbonyl group with a hydrogen atom on the end
ketones: consist of two alkyl groups attached to a central carbonyl group
naming aldehydes and ketones:
aldehyde: name ends with -al
ketones: name ends with -one
if the carbon chain has 5 or more carbon atoms a number is needed to indicate the location of the carbonyl group
properties of aldehydes and ketones:
lower boiling points and less soluble than corresponding alcohols
more soluble than corresponding alkanes
aldehydes and ketones can mix with both polar and non-polar substances
allows non-polar materials to be mixed with polar materials
preparing aldehydes and ketones from alcohols by oxidation reaction:
oxidation: reactions involve a loss of electrons
the element or compound that loses electrons is oxidized
the element or compound that gains electrons is reduced
controlled oxidation of alcohols results in aldehydes and ketones
the reactive oxygen atoms are supplied by oxidizing agents (O)
when primary alcohol is oxidized, an H atom remained on the C atom and an aldehyde is produced
primary alcohol + (O) → aldehyde + water
when secondary alcohol is oxidized, the carbonyl group that forms is attached to two alkyl groups forming a ketone
secondary alcohol + (O) → ketone + water
tertiary alcohols don’t react since there’s no hydrogen available for oxidation
hydration: hydrogen can be added to the carbonyl group in aldehydes and ketones
high temperatures and catalysts are required for this reaction
this is the reverse of the controlled oxidation of alcohols
Carboxylic Acids and Esters
carboxylic acid: characterized by the presence of carboxyl functional groups (R-COOH)
naming carboxylic acids:
the carboxyl group is made up of a hydroxyl (OH) bound to the C atom on a carbonyl group
the main chain is the longest chain containing the carboxyl group
ends with -oic acid
when naming multiple carboxyl groups the suffix -dioic is used
when more than two carboxyl groups are present, all COOH groups may be named as substituents on the parent chain
the parent chain doesn’t include the carboxylic atoms
properties of carboxylic acids:
highly polar molecules
the polarity of the carboxyl group makes carboxylic acids soluble in water
chains longer than ten carbons insoluble in water
higher boiling points than their corresponding alkanes
smaller members are soluble in water, larger carboxylic acids are relatively insoluble
conduct electricity
react with organic bases in neutralization reactions
short chain carboxylic acids are liquids at standard temperature
long chain carboxylic acids are waxy solids
preparing carboxylic acid:
when alcohol is mildy oxidized, an aldehyde is produced
if this aldehyde is oxidized further a carboxylic acid is produced
alcohol + (O) → aldehyde
aldehyde + (O) → carboxylic acid
ester: similar to carboxylic acids but th eH atom in the acid is replaced with another alkyl group (R - COO - R)
naming esters:
the name of an ester has 2 parts
first part: the name of the alkyl group used in the esterification process
second part: the name of the acid
the ending of the acid name change from -oic acid to -oate
properties of esters:
the presence of the carbonyl group make esters somewhat polar
esters are less polar than corresponding carboxylic acids because they lack an OH group capable of H bonding
they are less soluble in water with lower melting and boiling points than corresponding alcohols and carboxylic acids
smaller esters are liquid at standard temperatures while longer esters are insoluble
reactions of esters:
esterification: carboxylic acid + alcohol → ester + water
alcohol acts as an organic base and carboxylic acid acts as an acid
the ester formed is considered an organic salt
hydrolysis: reverses esterification by reacting the ester with an acid or base
ester + acid/base → acid + alcohol
a bond is broken by the addition of water resulting in two or more products
Amines and Amides
amines: ammonia with one to all of its hydrogens substituted by alkyl groups
there are three classifications of amines:
primary amine: one hydrogen is substituted with an alkyl group
prepared by reacting ammonia with an alkyl halide
ammonia + alkyl halide → primary amine + hydrogen halide
secondary amine: two hydrogens are substituted with alkyl groups
prepared by reacting a primary amine further with an alkyl halide
primary amine + alkyl halide → secondary amine + alkyl halide
tertiary amine: all three hydrogens are substituted with alkyl groups
prepared by reacting a secondary amine further with an alkyl halide
secondary amine + alkyl halide → tertiary amine + alkyl halide
naming amines:
amines are nitrogen derivatives of an alkane
amines can also be named as alkyl derivatives of ammonia
diamines: molecules with 2 amino groups
for secondary and tertiary amines include the N-prefix to show the substituted groups on the amine
properties of amines:
primary and secondary amines are very polar due to the N-H bond which allows them to H-bond with each other
tertiary amines don’t have N-H bonds and can’t H-bond
higher boiling and melting points than similar-sized ethers and alkanes
smaller amines are soluble in water
N-C and N-H bonds are more polar
amines share a lower boiling point than alcohols of similar size because N-H bonds are less polar than O-H bonds
when amines are created using alkyl halides, a mixture of primary, secondary, and tertiary amines result
can separate the different amines through boiling point
amides: hydrocarbon that contains a carbonyl group bonded to a nitrogen atom
similar to esters but the N atom replaces the O atom in the chain of an ester
they are the backbone of all protein molecules
in proteins amide bonds are called peptides
properties of amides:
amides have a polar carbonyl group and amides with a least one NH group can form strong hydrogen bonds among themselves
higher boiling points that their corresponding hydrocarbon derivatives
weak bases that are insoluble in water
low molecular weight amides are slightly soluble
can be hydrolyzed in acidic or basic conditions to produce a carboxylic acid and an amine
naming amides:
the first part of an amide’s name comes from the amine
the second part of the name comes from the acid
ends with the suffix -amide
if one or more alkyl groups is attached to the N atom the upper case N is used to clarify the location
preparing amides:
condensation: carboxylic acids react with ammonia or primary or secondary amines to produce amides
carboxylic acid + ammonia → amide + water
tertiary amines don’t undergo condensation reactions since they lack the extra H atoms needed to make water
amides can also be made with primary amines and carboxylic acids by condensation reactions
carboxylic acid + primary amine → amide + water
Synthetic Addition Polymers
polymer: made up of a group of monomers (usually 10 or more)
monomer: a hydrocarbon derived molecule
polymers may contain thousands of individual monomers, the subscript n is used to indicate the number of repeating units
polyethylene: a polymer of ethene
under certain conditions alkenes undergo addition reactions with other alkenes
the double bond in each alkene changes into a single bond freeing an unbonded electron to form a single bond with other ethene monomers
addition polymerization: consists of three stages (initiation, propagation, and termination)
an initiating molecule with an unpaired electron forms a bond with one of the carbonatoms in the double bonded monomer
this yields an unpaired electron on the other end of the monomer
this electron can then form covalen bonds with another group
the reaction continues/propigates
the chain grows until two unpaired electrons combine forming a covalent bond that links the growing chains together
properties of plastics:
plastics are chemically unreactive because chemically active unsaturated alkenes have been converted into unreactive saturated carbon chains
the strong covalent bonds make the structure very stable
most intermolecular forces are Van der waals forces but because the molecules are so large these forces are very strong
strengthening polymers with cross-linking:
alkenes with double bonds (-dienes) are monomers for several polymers
these polymers often end in -ene
cross-linking occurs between -dienes because the second double bond allows for the formation of covalent bonds between different chains of monomers
Synthetic Condensation Polymers
dimers: formed with molecules with functional groups react with other molecules
condemnation polymers: when monomers join end to end in ester or amide linkages they form polyesters and polyamides
polyesters: a polymer of carboxylic acids and alcohols
when condensation reactions are repeatedly used to join carboxylic acids and alcohols, a polyester is formed
uses a dicarboxylic acid and a diol
the acids donates the OH, the alcohol donates the H
polyamides: a polymer of carboxylic acids and amines
they are condensation polymers consisting of many amides
the monomers must contain carboxyl groups, amine groups, or one of each
 Knowt
Knowt