
1.1- 1.3 Solids Liquids Gases
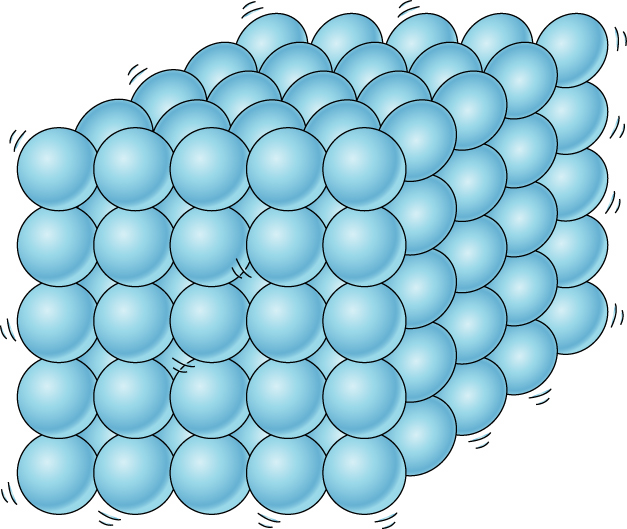
Solids
Solids have a fixed volume and shape and they have a high density
The atoms vibrate in a fixed position but can’t move around
The particles are packed very closely together in a fixed and regular pattern
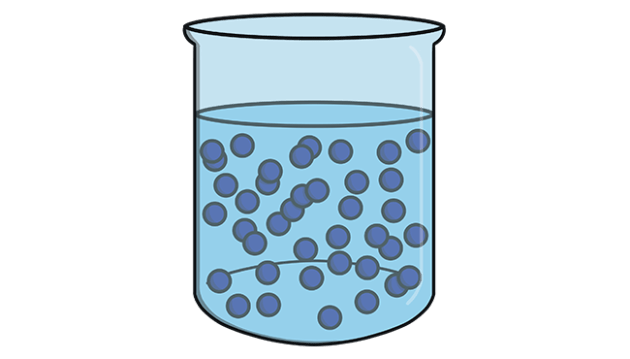
Liquids
Liquids also have a fixed volume but take the shape of the container
They are generally less dense than solids (an exception is water), but much denser than gases
The particles move and slide past each other which is why liquids adopt the shape of the container and also why they are able to flow freely
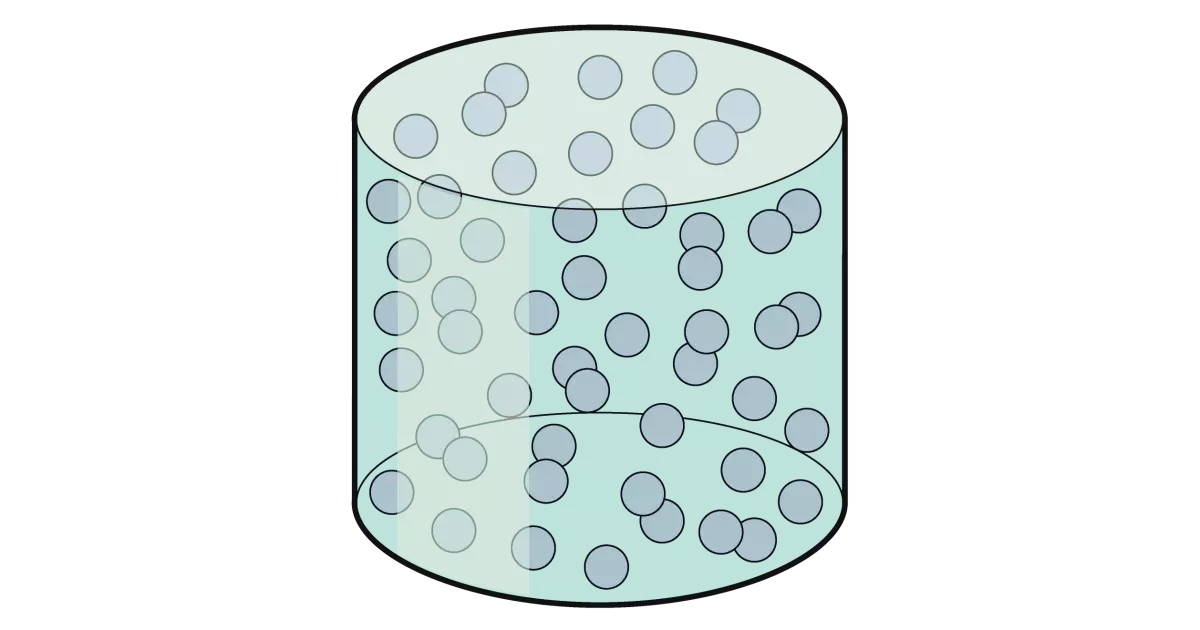
Gases
Gases do not have a fixed volume, and, like liquids, take up the shape of the container
Gases have a very low density
Since there is a lot of space between the particles, gases can be compressed into a much smaller volume
The particles are far apart and move randomly and quickly
They collide with each other and with the sides of the container (this is how pressure is created inside a can of gas)
1.2 Particles are Everywhere.
Rock air and water look very different but they have one thing in common they are made of tiny pieces. let’s call these Particles.
Everything around you is made up of Particles, in-fact you Yourself are made of particles!
Proof from Pollen
For centuries, people had guessed that water and air were made of tiny particles. But since they could note see them, they could not prove it.
Then in 1872, a botanist caller Robert Brown noticed something strange. He was studying pollen from a flower, in water under a microscope. He saw particles from the pollen jiggling around, They were not alive- so why were they moving?
78 years later, Albert Einstein came up with the answer. The particles moved because they were being struck by tiny invisible moving water particles. In-fact their movement proved that water is made of particles.
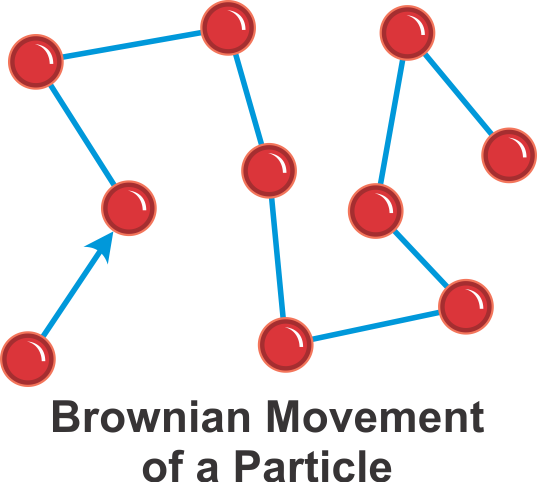
Brownian Motion:
The Random Movement of particles that you see with the naked eyes or under the microscope is called Brownian motion.
The particles follow a Zig Zag path because they are being struck by tiny invisible particles.
Further Evidence of the Brownian Motion:
In a Sunlit rooms you sometimes see dust dancing in the air. It dances because the dust particles are being bombarded by the tiny moving particles in air this is an example of Brownian motion.

Cooking smells spread. The ‘smells’ are due to tiny particles, which spread because they are bing born added by the particles in air, kind of like diffusion.
Place a Crystal of purple potassium manganate (VII) in a beaker of water. The color spreads through the water, because the particles leave the solid crystal- it dissolves and spread among the water particles

in example 1, 2, and 3, we cannot see the moving particles-even with the most powerful microscopes. They are far too small. so what are they??
The smallest particles, that we cannot not break down further in chemical reactions, are called atoms In some substances, the particles consist of two or more atoms joined together. These particles are called molecules. Water and bromine exist as molecules. Air is mostly nitrogen and oxygen molecules.
In other substances the particles are atoms or groups of atoms that carry charge. these particles are called ions. In example 3 above, the particles in potassium manganate (VII) are ions.
But what about the particles Robert Brown observed? They contained 1000 atoms. That’s why they were big enough to be seen with microscope. Dust Particles in air contain thousands of atoms too.
1.3 Changing states of matter
Melting
Melting is when a solid changes into a liquid
Requires heat energy which transforms into kinetic energy, allowing the particles to move
Occurs at a specific temperature known as the melting point (m.p.)
Boiling
Boiling is when a liquid changes into a gas
Requires heat which causes bubbles of gas to form below the surface of a liquid, allowing for liquid particles to escape from the surface and within the liquid
Occurs at a specific temperature known as the boiling point (b.p.)
Freezing
Freezing is when a liquid changes into a solid
This is the reverse of melting and occurs at exactly the same temperature as melting, hence the melting point and freezing point of a pure substance are the same. Water, for example, freezes and melts at 0 ºC
Requires a significant decrease in temperature (or loss of thermal energy) and occurs at a specific temperature
Evaporation
Evaporation occurs when a liquid changes into a gas and occurs over a range of temperatures
Evaporation occurs only at the surface of liquids where high energy particles can escape from the liquid's surface at low temperatures, below the b.p. of the liquid
The larger the surface area and the warmer the liquid surface, the more quickly a liquid can evaporate
Condensation
Condensation occurs when a gas changes into a liquid on cooling and it takes place over a range of temperatures
When a gas is cooled its particles lose energy and when they bump into each other they lack the energy to bounce away again, instead they group together to form a liquid
Sublimation
When a solid changes directly into a gas, without going through the liquid stage
This happens to only a few solids such as iodine or solid carbon dioxide
The reverse reaction also happens and is called deposition or desublimation
Changes in the State and the Kinetic theory
When substances are heated, the particles absorb thermal energy which is converted into kinetic energy. This is the basis of the kinetic theory of matter
Heating a solid causes its particles to vibrate more and as the temperature increases, they vibrate so much that the solid expands until the structure breaks and the solid melts
On further heating, the now liquid substance expands more and some particles at the surface gain sufficient energy to overcome the intermolecular forces and evaporate
When the b.p. temperature is reached, all the particles gain enough energy to escape and the liquids boils
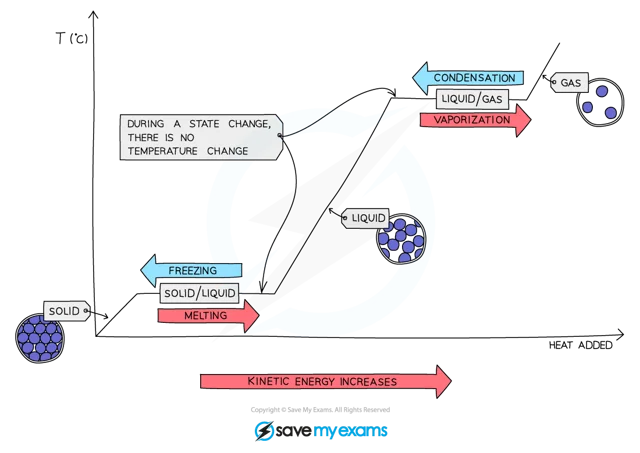
These changes in state can be shown on a graph called a heating curve
Cooling down a gas has the reverse effect and this would be called a cooling curve
These curves are used to show how changes in temperature affect changes of state
Pressure Temperature and Gas
Gaseous particles are in constant and random motion
An increase in temperature increases the kinetic energy of each particle, as the heat energy is transformed to kinetic energy, so they move faster
Decreasing the temperature has the opposite effect, so the particles slow down
As the temperature increases, the particles in the gas move faster, impacting the container's walls more frequently
The pressure that a gas creates inside a closed container is produced by the gaseous particles hitting the inside walls of the container
So when there is an increase in temperature this also causes an increase in gas pressure
increase in temperature = increase in kinetic energy of particle = increase in movement of particles
1.1- 1.3 Solids Liquids Gases
Solids
Solids have a fixed volume and shape and they have a high density
The atoms vibrate in a fixed position but can’t move around
The particles are packed very closely together in a fixed and regular pattern

Liquids
Liquids also have a fixed volume but take the shape of the container
They are generally less dense than solids (an exception is water), but much denser than gases
The particles move and slide past each other which is why liquids adopt the shape of the container and also why they are able to flow freely

Gases
Gases do not have a fixed volume, and, like liquids, take up the shape of the container
Gases have a very low density
Since there is a lot of space between the particles, gases can be compressed into a much smaller volume
The particles are far apart and move randomly and quickly
They collide with each other and with the sides of the container (this is how pressure is created inside a can of gas)

1.2 Particles are Everywhere.
Rock air and water look very different but they have one thing in common they are made of tiny pieces. let’s call these Particles.
Everything around you is made up of Particles, in-fact you Yourself are made of particles!
Proof from Pollen
For centuries, people had guessed that water and air were made of tiny particles. But since they could note see them, they could not prove it.
Then in 1872, a botanist caller Robert Brown noticed something strange. He was studying pollen from a flower, in water under a microscope. He saw particles from the pollen jiggling around, They were not alive- so why were they moving?
78 years later, Albert Einstein came up with the answer. The particles moved because they were being struck by tiny invisible moving water particles. In-fact their movement proved that water is made of particles.
Brownian Motion:
The Random Movement of particles that you see with the naked eyes or under the microscope is called Brownian motion.
The particles follow a Zig Zag path because they are being struck by tiny invisible particles.

Further Evidence of the Brownian Motion:
In a Sunlit rooms you sometimes see dust dancing in the air. It dances because the dust particles are being bombarded by the tiny moving particles in air this is an example of Brownian motion.

Cooking smells spread. The ‘smells’ are due to tiny particles, which spread because they are bing born added by the particles in air, kind of like diffusion.
Place a Crystal of purple potassium manganate (VII) in a beaker of water. The color spreads through the water, because the particles leave the solid crystal- it dissolves and spread among the water particles

in example 1, 2, and 3, we cannot see the moving particles-even with the most powerful microscopes. They are far too small. so what are they??
The smallest particles, that we cannot not break down further in chemical reactions, are called atoms In some substances, the particles consist of two or more atoms joined together. These particles are called molecules. Water and bromine exist as molecules. Air is mostly nitrogen and oxygen molecules.
In other substances the particles are atoms or groups of atoms that carry charge. these particles are called ions. In example 3 above, the particles in potassium manganate (VII) are ions.
But what about the particles Robert Brown observed? They contained 1000 atoms. That’s why they were big enough to be seen with microscope. Dust Particles in air contain thousands of atoms too.
1.3 Changing states of matter
Melting
Melting is when a solid changes into a liquid
Requires heat energy which transforms into kinetic energy, allowing the particles to move
Occurs at a specific temperature known as the melting point (m.p.)
Boiling
Boiling is when a liquid changes into a gas
Requires heat which causes bubbles of gas to form below the surface of a liquid, allowing for liquid particles to escape from the surface and within the liquid
Occurs at a specific temperature known as the boiling point (b.p.)
Freezing
Freezing is when a liquid changes into a solid
This is the reverse of melting and occurs at exactly the same temperature as melting, hence the melting point and freezing point of a pure substance are the same. Water, for example, freezes and melts at 0 ºC
Requires a significant decrease in temperature (or loss of thermal energy) and occurs at a specific temperature
Evaporation
Evaporation occurs when a liquid changes into a gas and occurs over a range of temperatures
Evaporation occurs only at the surface of liquids where high energy particles can escape from the liquid's surface at low temperatures, below the b.p. of the liquid
The larger the surface area and the warmer the liquid surface, the more quickly a liquid can evaporate
Condensation
Condensation occurs when a gas changes into a liquid on cooling and it takes place over a range of temperatures
When a gas is cooled its particles lose energy and when they bump into each other they lack the energy to bounce away again, instead they group together to form a liquid
Sublimation
When a solid changes directly into a gas, without going through the liquid stage
This happens to only a few solids such as iodine or solid carbon dioxide
The reverse reaction also happens and is called deposition or desublimation
Changes in the State and the Kinetic theory
When substances are heated, the particles absorb thermal energy which is converted into kinetic energy. This is the basis of the kinetic theory of matter
Heating a solid causes its particles to vibrate more and as the temperature increases, they vibrate so much that the solid expands until the structure breaks and the solid melts
On further heating, the now liquid substance expands more and some particles at the surface gain sufficient energy to overcome the intermolecular forces and evaporate
When the b.p. temperature is reached, all the particles gain enough energy to escape and the liquids boils
These changes in state can be shown on a graph called a heating curve
Cooling down a gas has the reverse effect and this would be called a cooling curve
These curves are used to show how changes in temperature affect changes of state

Pressure Temperature and Gas
Gaseous particles are in constant and random motion
An increase in temperature increases the kinetic energy of each particle, as the heat energy is transformed to kinetic energy, so they move faster
Decreasing the temperature has the opposite effect, so the particles slow down
As the temperature increases, the particles in the gas move faster, impacting the container's walls more frequently
The pressure that a gas creates inside a closed container is produced by the gaseous particles hitting the inside walls of the container
So when there is an increase in temperature this also causes an increase in gas pressure
increase in temperature = increase in kinetic energy of particle = increase in movement of particles
 Knowt
Knowt
