
Chapter 2: Cell Chemistry and Bioenergetics
Cell Chemistry and Bioenergetics
Until the 19th century, people knew “Vital Force” to be responsible for all the distinctive properties.
Organic Chemistry: It is the study of hydrocarbons and their derivatives.
Why chemistry of life is indeed special?
Firstly, it is based on carbon compounds.
Secondly, cells are 70% water, and life depends largely on chemical reactions.
Thirdly, cell chemistry is enormously complex.
Chemical components of cell:
It is made up of mainly four elements including carbon(C), hydrogen(H), nitrogen(N), and oxygen(O).
A cell is formed from carbon compounds.
Carbon has a property of catenation which helps in the self-linking of atoms of an element to form chains and rings.
Carbon has a covalency of four.
It confers stability to form large molecules.
A cell also contains small organic molecules:
Sugars
Fatty Acids
Nucleotides
Amino Acids
Types of bonds:
Covalent Bond: A chemical bond that involves the sharing of electrons to form electron pairs between atoms.
Non-Covalent Bond: The bond in which no sharing of electron pairs takes place is called a non-covalent bond.
Hydrogen Bond: A hydrogen bond is the interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen, or fluorine from another molecule.
Non-Covalent attractions:
Electrostatic attractions (ionic bonds)
Hydrogen Bonds
Vander Waal attractions
Hydrophobic force
Acids: Substances that release protons when they dissolve in water thus forming H3O+ are termed acids.
Strong Acids: Those who lose their protons quickly. Eg: Hydrochloric acid (HCL).
Weak Acids: Those who hold on to their proton more tightly when dissolved in water. Eg: Acetic Acid (C2H5COOH)
Bases: The opposite of acid is a base. Substances that accept a proton from a water molecule are called bases.
Strong Base: Those who readily dissociate in water to form respective ions. Eg: Sodium Hydroxide (NaOH)
Weak Base: Those who have a weak tendency to reversibly accept a proton from water. Eg: Ammonia (NH3)
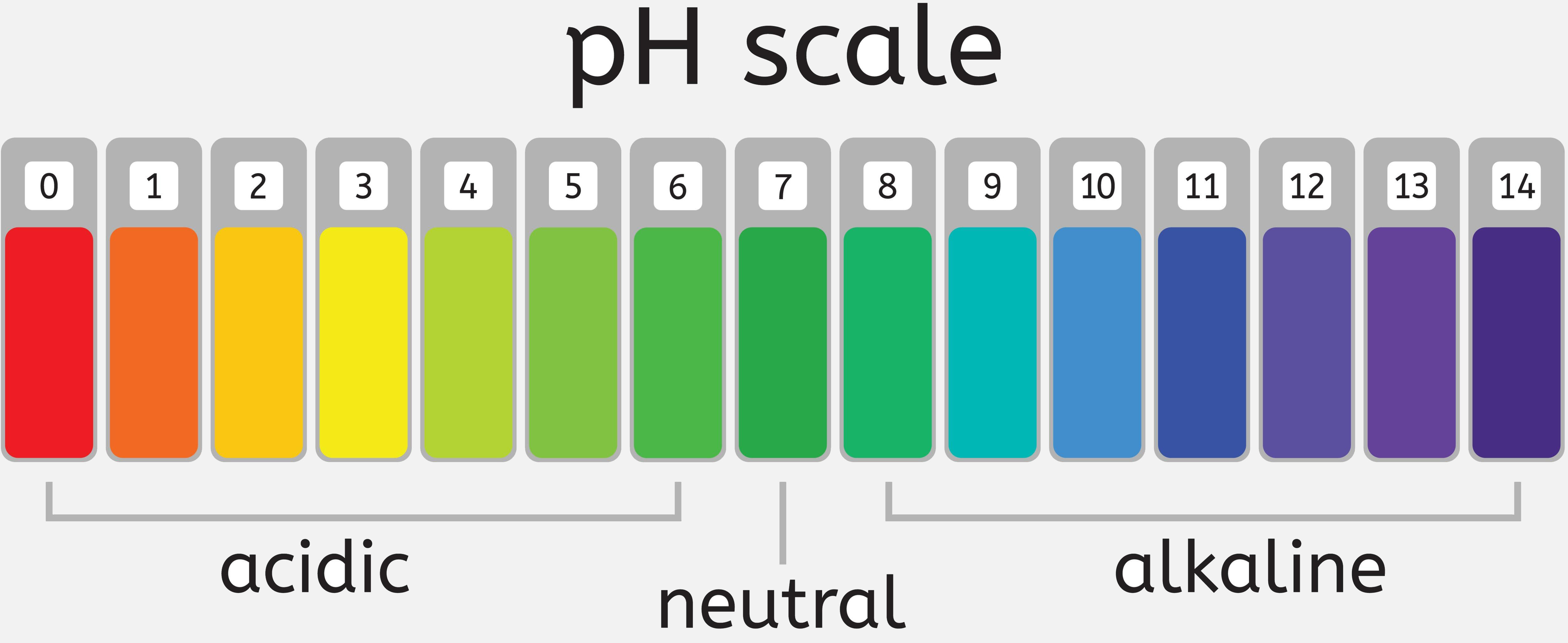
pH Scale: The concentration of H3O+ is expressed using a logarithmic scale called as pH scale.
Pure water has a pH of 7.0.
An acidic solution has a pH of <7.
The basic solution has a pH of >7.
Buffers: It is a solution that can resist pH change upon the addition of an acidic or basic component.
Types of molecules:
Macromolecule: It is composed of a much larger number of atoms than ordinary molecules.
Micromolecule: It is a small molecule that often joins together to form a larger type of molecule. It is often referred to as monomers.
Cell Metabolism
It is the set of chemical reactions that occur to maintain life.
Metabolism = Catabolism + Anabolism
Catabolic Reactions: These reactions break down molecules into smaller units.
Anabolic Reactions: These reactions use the small molecules and the energy harnessed by catabolism to drive the synthesis of molecules.
Thermodynamics: (branch of science which deals with the energy changes taking place in all physical and chemical processes)
Thermo (heat) + Dynamics (flow/motion)
Key terms:
Work: The product of force and displacement is called work
w = (- P ΔV )
w= work done; P= pressure; ΔV = change in volume
System: It is any region of space that is under thermodynamic investigation.
Open system: This type of system can exchange energy as well as matter with the surrounding.
Closed system: This type of system can exchange energy, but not matter with the surroundings.
Surrounding: It comprises the rest of the universe apart from the system.
Universe: It comprises the system and its surroundings together.
Boundary: A wall or layer separating the surrounding.
A boundary can be rigid or non-rigid.
A boundary can be conducting or non-conducting.
A boundary can be real or imaginary.
Internal Energy (E): It is defined as the sum of different energies associated with its atoms and molecules.
Laws of thermodynamics:
The first law of thermodynamics:
This law is based on the law of conservation of energy.
Energy can neither be created nor be destroyed but can be transformed from one form to another.
The total energy of the universe is always constant.
ΔE= q + w (a mathematical form of 1st law of thermodynamics)
q: energy given to the system; w: work done on the system; ΔE: change in internal energy.
Enthalpy (H): Heat contained in the system measured at constant pressure. {H = E + PV}
The second law of thermodynamics: States that in the universe or any isolated system the degree of disorder always increases.
Spontaneity: It defines whether a chemical reaction will occur or not.
Causes of spontaneity:
Decrease in potential energy (stored energy at rest).
Increase in randomness or disorder.
Reactions proceeds in that direction where randomness increase.
Reactions are of two types:
Spontaneous Reaction: Reaction which can occur by itself without any external force.
Non-Spontaneous Reaction: Reaction which cannot occur by itself.
Entropy: It is a measure of randomness or disorder in a system. The greater the disorder, the greater the entropy.
Gibb’s Energy (G): It is the part of the total energy of the system which can be converted to useful work. (∆G = ∆H -- T∆S)
∆G: change in Gibbs energy; ∆H: change in enthalpy; ∆S: change in entropy.
For a reaction Y → X at 37°C, ∆G° is related to ∆G as follows:
∆G = ∆G° + RT ln [X] /[Y]
Relationship between standard Gibb’s energy change (∆G°) and Equilibrium Constant (Keq):
∆G° = - RT ln Keq
Key terms for different energy reactions:
Oxidation: It is the loss of electrons or an increase in the oxidation state of a chemical or atoms within it.
Reduction: It is the gain of electrons or a decrease in the oxidation state of a chemical or atoms within it.
Hydrogenation: It is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst.
Dehydrogenation: It is the process by which hydrogen is removed from an organic compound to form a new compound.
Activation Energy: It is the minimum amount of energy that must be provided for compounds to result in a chemical reaction.
Enzymes: A substance produced by a living organism that acts as a catalyst to bring about a specific biochemical reaction.
Coenzymes: Coenzymes are small molecules. They cannot by themselves catalyze a reaction but they can help enzymes to do so.
Substrates: Each enzyme binds tightly to one or more molecules called substrates.
Catalysts: A substance that can lower/increase the activation energy of a reaction.
Important Abbreviations to understand the different processes:
ATP: Adenosine Tri Phosphate
ADP: Adenosine Di Phosphate
NADH: Nicotinamide Adenine Dinucleotide
NADPH: Nicotinamide Adenine Dinucleotide Phosphate
FADH2: Flavin Adenine Dinucleotide
AMP: Adenosine Mono Phosphate
PPi: Pyrophosphate
How do cells obtain energy from food?
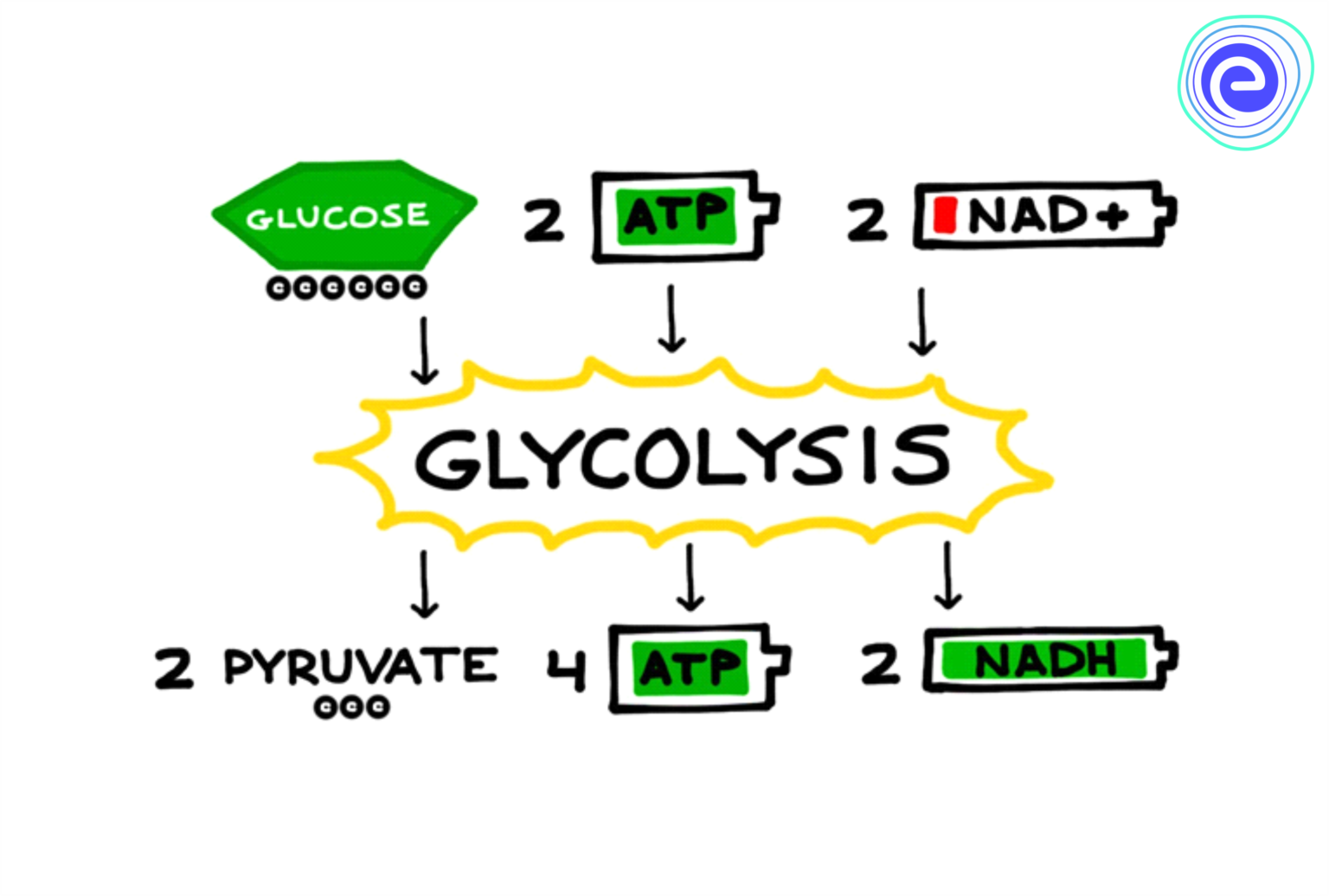
Glycolysis: The major process of oxidizing sugars is the sequence of reactions known as glycolysis.
It is common in both aerobic (in presence of oxygen) and anaerobic (without the presence of oxygen) reactions.
It takes place in the cytoplasm of the cell.
It starts with 6-carbon glucose to finally result in two molecules of 3-C pyruvate. In plants, this glucose is derived from sucrose.
Total ATP produced: 8ATP
Fermentation:
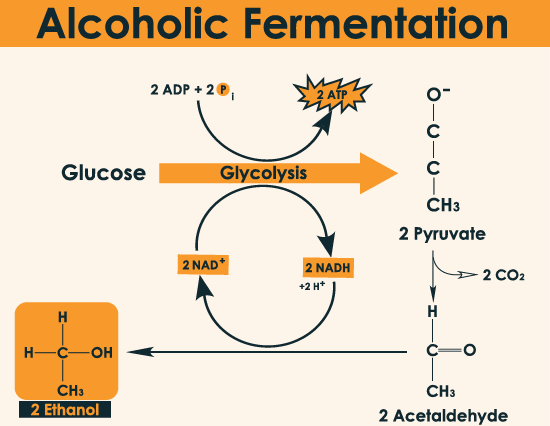
Alcoholic Fermentation:
It occurs in yeast.
The process is hazardous either acid or alcohol is produced. Yeats poison themselves to death when the concentration reaches about 13%.
It yields ethyl alcohol as the final product.
Total ATP produced: 2ATP
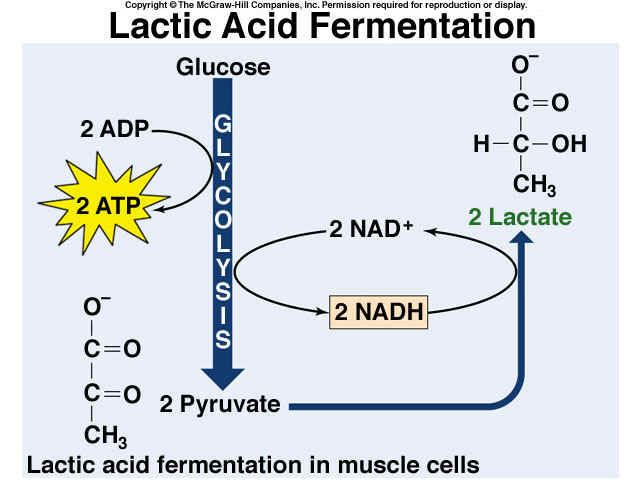
Lactic Acid Fermentation:
It occurs in the muscles of humans during an intense workout.
it yields lactic acid as the final product.
Total ATP produced: 2ATP
Oxidative Phosphorylation:
The process that connects glycolysis and Krebs’s Cycle.
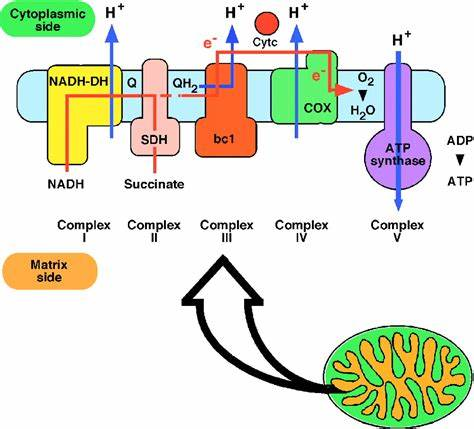
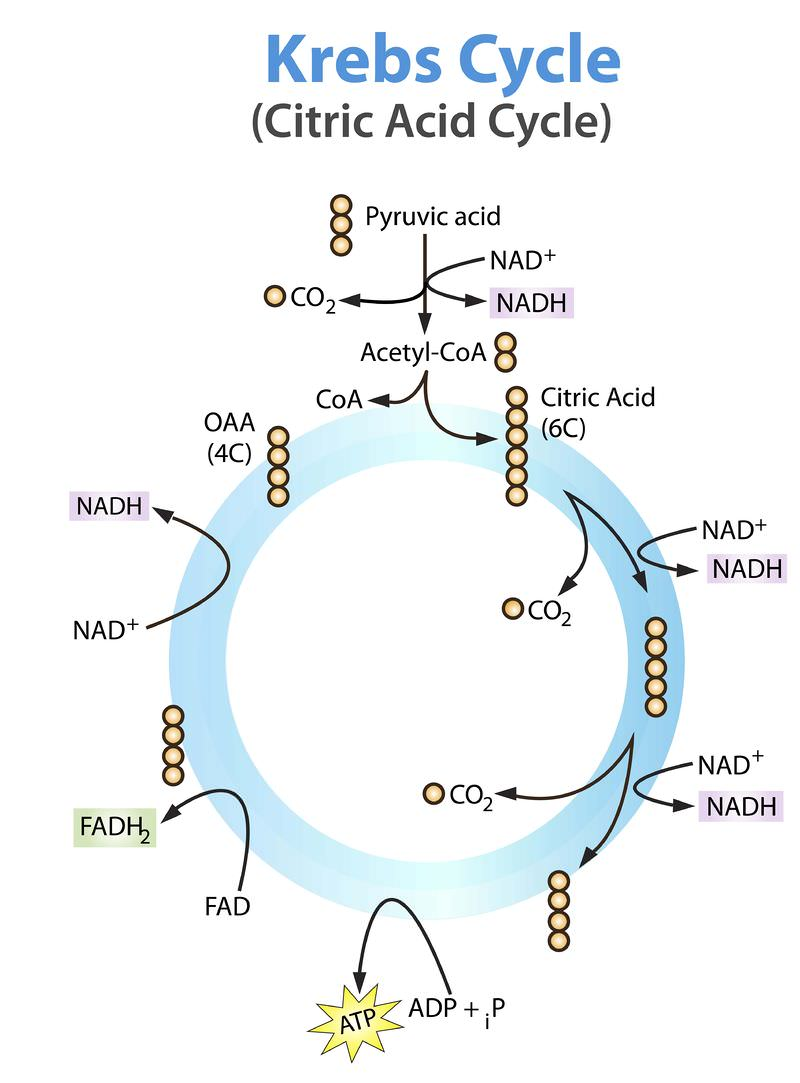
Krebs’s Cycle:
It occurs in the mitochondria matrix of the eukaryotic cell but in prokaryotes, it occurs in the cytoplasm.
Stored food in organisms:
Fungi: Oil and glycogen
Humans: Glycogen
Plants: Starch
Chapter 2: Cell Chemistry and Bioenergetics
Cell Chemistry and Bioenergetics
Until the 19th century, people knew “Vital Force” to be responsible for all the distinctive properties.
Organic Chemistry: It is the study of hydrocarbons and their derivatives.
Why chemistry of life is indeed special?
Firstly, it is based on carbon compounds.
Secondly, cells are 70% water, and life depends largely on chemical reactions.
Thirdly, cell chemistry is enormously complex.
Chemical components of cell:
It is made up of mainly four elements including carbon(C), hydrogen(H), nitrogen(N), and oxygen(O).
A cell is formed from carbon compounds.
Carbon has a property of catenation which helps in the self-linking of atoms of an element to form chains and rings.
Carbon has a covalency of four.
It confers stability to form large molecules.
A cell also contains small organic molecules:
Sugars
Fatty Acids
Nucleotides
Amino Acids
Types of bonds:
Covalent Bond: A chemical bond that involves the sharing of electrons to form electron pairs between atoms.
Non-Covalent Bond: The bond in which no sharing of electron pairs takes place is called a non-covalent bond.
Hydrogen Bond: A hydrogen bond is the interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen, or fluorine from another molecule.
Non-Covalent attractions:
Electrostatic attractions (ionic bonds)
Hydrogen Bonds
Vander Waal attractions
Hydrophobic force
Acids: Substances that release protons when they dissolve in water thus forming H3O+ are termed acids.
Strong Acids: Those who lose their protons quickly. Eg: Hydrochloric acid (HCL).
Weak Acids: Those who hold on to their proton more tightly when dissolved in water. Eg: Acetic Acid (C2H5COOH)
Bases: The opposite of acid is a base. Substances that accept a proton from a water molecule are called bases.
Strong Base: Those who readily dissociate in water to form respective ions. Eg: Sodium Hydroxide (NaOH)
Weak Base: Those who have a weak tendency to reversibly accept a proton from water. Eg: Ammonia (NH3)
pH Scale: The concentration of H3O+ is expressed using a logarithmic scale called as pH scale.
Pure water has a pH of 7.0.
An acidic solution has a pH of <7.
The basic solution has a pH of >7.

Buffers: It is a solution that can resist pH change upon the addition of an acidic or basic component.
Types of molecules:
Macromolecule: It is composed of a much larger number of atoms than ordinary molecules.
Micromolecule: It is a small molecule that often joins together to form a larger type of molecule. It is often referred to as monomers.
Cell Metabolism
It is the set of chemical reactions that occur to maintain life.
Metabolism = Catabolism + Anabolism
Catabolic Reactions: These reactions break down molecules into smaller units.
Anabolic Reactions: These reactions use the small molecules and the energy harnessed by catabolism to drive the synthesis of molecules.
Thermodynamics: (branch of science which deals with the energy changes taking place in all physical and chemical processes)
Thermo (heat) + Dynamics (flow/motion)
Key terms:
Work: The product of force and displacement is called work
w = (- P ΔV )
w= work done; P= pressure; ΔV = change in volume
System: It is any region of space that is under thermodynamic investigation.
Open system: This type of system can exchange energy as well as matter with the surrounding.
Closed system: This type of system can exchange energy, but not matter with the surroundings.
Surrounding: It comprises the rest of the universe apart from the system.
Universe: It comprises the system and its surroundings together.
Boundary: A wall or layer separating the surrounding.
A boundary can be rigid or non-rigid.
A boundary can be conducting or non-conducting.
A boundary can be real or imaginary.
Internal Energy (E): It is defined as the sum of different energies associated with its atoms and molecules.
Laws of thermodynamics:
The first law of thermodynamics:
This law is based on the law of conservation of energy.
Energy can neither be created nor be destroyed but can be transformed from one form to another.
The total energy of the universe is always constant.
ΔE= q + w (a mathematical form of 1st law of thermodynamics)
q: energy given to the system; w: work done on the system; ΔE: change in internal energy.
Enthalpy (H): Heat contained in the system measured at constant pressure. {H = E + PV}
The second law of thermodynamics: States that in the universe or any isolated system the degree of disorder always increases.
Spontaneity: It defines whether a chemical reaction will occur or not.
Causes of spontaneity:
Decrease in potential energy (stored energy at rest).
Increase in randomness or disorder.
Reactions proceeds in that direction where randomness increase.
Reactions are of two types:
Spontaneous Reaction: Reaction which can occur by itself without any external force.
Non-Spontaneous Reaction: Reaction which cannot occur by itself.
Entropy: It is a measure of randomness or disorder in a system. The greater the disorder, the greater the entropy.
Gibb’s Energy (G): It is the part of the total energy of the system which can be converted to useful work. (∆G = ∆H -- T∆S)
∆G: change in Gibbs energy; ∆H: change in enthalpy; ∆S: change in entropy.
For a reaction Y → X at 37°C, ∆G° is related to ∆G as follows:
∆G = ∆G° + RT ln [X] /[Y]
Relationship between standard Gibb’s energy change (∆G°) and Equilibrium Constant (Keq):
∆G° = - RT ln Keq
Key terms for different energy reactions:
Oxidation: It is the loss of electrons or an increase in the oxidation state of a chemical or atoms within it.
Reduction: It is the gain of electrons or a decrease in the oxidation state of a chemical or atoms within it.
Hydrogenation: It is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst.
Dehydrogenation: It is the process by which hydrogen is removed from an organic compound to form a new compound.
Activation Energy: It is the minimum amount of energy that must be provided for compounds to result in a chemical reaction.
Enzymes: A substance produced by a living organism that acts as a catalyst to bring about a specific biochemical reaction.
Coenzymes: Coenzymes are small molecules. They cannot by themselves catalyze a reaction but they can help enzymes to do so.
Substrates: Each enzyme binds tightly to one or more molecules called substrates.
Catalysts: A substance that can lower/increase the activation energy of a reaction.
Important Abbreviations to understand the different processes:
ATP: Adenosine Tri Phosphate
ADP: Adenosine Di Phosphate
NADH: Nicotinamide Adenine Dinucleotide
NADPH: Nicotinamide Adenine Dinucleotide Phosphate
FADH2: Flavin Adenine Dinucleotide
AMP: Adenosine Mono Phosphate
PPi: Pyrophosphate
How do cells obtain energy from food?
Glycolysis: The major process of oxidizing sugars is the sequence of reactions known as glycolysis.
It is common in both aerobic (in presence of oxygen) and anaerobic (without the presence of oxygen) reactions.
It takes place in the cytoplasm of the cell.
It starts with 6-carbon glucose to finally result in two molecules of 3-C pyruvate. In plants, this glucose is derived from sucrose.
Total ATP produced: 8ATP

Fermentation:
Alcoholic Fermentation:
It occurs in yeast.
The process is hazardous either acid or alcohol is produced. Yeats poison themselves to death when the concentration reaches about 13%.
It yields ethyl alcohol as the final product.
Total ATP produced: 2ATP

Lactic Acid Fermentation:
It occurs in the muscles of humans during an intense workout.
it yields lactic acid as the final product.
Total ATP produced: 2ATP

Oxidative Phosphorylation:
The process that connects glycolysis and Krebs’s Cycle.

Krebs’s Cycle:
It occurs in the mitochondria matrix of the eukaryotic cell but in prokaryotes, it occurs in the cytoplasm.

Stored food in organisms:
Fungi: Oil and glycogen
Humans: Glycogen
Plants: Starch
 Knowt
Knowt