
Chapter 13: Global Change
Global climate change, ozone depletion, ocean warming, and endangered species are just a few of the ways humans have impacted the Earth.
You will need to look at global changes and be able to explain the causes and consequences of these. Make sure you can look at data and use this data to propose solutions or legislation to global problems.
One of the most important aspects that students confuse is the difference between global climate change and ozone depletion.
Local and regional human activities can have impacts at the global level.
The health of a species is closely tied to its ecosystem, and minor environmental changes can have a large impact.
Key Terms
Stratospheric ozone layer: The protected layer of our atmosphere that absorbs UV light.
Chlorofluorocarbons: A man-made chemical that works its way through the troposphere and into the stratosphere where it can destroy ozone molecules.
Ozone depletion: caused by chemicals like CFCs breaking down ozone in the stratosphere. This means less natural ozone to absorb UV light coming to Earth.
Greenhouse effect: The natural ability of Earth to absorb heat from the sun (infrared) and keep our planet warm enough to live on.
Global climate change: As more and more greenhouse gases are emitted into the atmosphere, we are trapping more heat from the sun and the temperature of the Earth is getting warmer. Sea levels are rising, estuaries are getting deeper, storms get stronger, insects are moving farther away from the equator, ice caps are melting, and permafrost is melting.
Positive feedback loop: When one thing causes something else to get progressively worse.
Coral bleaching: When the algae that lives in the coral animals dies or leaves the coral and causes the coral to die and turn white.
Ocean warming: When the Earth is getting warmer the oceans are also getting warmer. This is causing ice to melt, sea levels to rise, etc.
Ocean acidification: As more carbon dioxide is added to the atmosphere it gets into the ocean causing the ocean to have a lower pH and harming things like coral animals.
Invasive species: Nonnative species that are introduced to an environment and might outcompete the native species.
Endangered species: Any species that is threatened to go extinct.
Habitat fragmentation: When a habitat is split up into smaller areas with roads, homes, and more in the middle, it leads to loss of biodiversity.
Stratospheric Ozone Depletion
The stratospheric ozone layer is a shield around the Earth that absorbs most of the sun’s ultraviolet radiation (UV).
This layer of our atmosphere contains ozone (O3). It is the ozone (O3) and oxygen molecules (O2) in this layer that absorb the UV light.
The stratosphere is above the troposphere, which is the layer closest to the Earth’s surface; it extends from 10 km (6 miles) to about 50 km (31 miles) into space.
Because of the stratosphere’s ability to absorb the sun’s UV light, this dangerous radiation is prevented from passing to the Earth’s surface.
Without this stratospheric ozone layer life on Earth as we know it would not be possible.
Ozone is produced naturally in the stratosphere when the sun’s UV rays split O2 molecules into single oxygen atoms.
These oxygen atoms then combine with O2 to form O3 molecules, which are very good at absorbing UV light.
However, human-produced chemicals, such as chlorofluorocarbons (CFCs), although they are heavier than air, have been carried into the stratosphere by the air currents and mixing processes of the atmosphere.
These CFC molecules are hit by the sun’s UV energy and break up, releasing chlorine atoms.
The chlorine atoms then react with the O3 molecules, breaking up the O3 by taking one oxygen atom to form chlorine monoxide and O2.
The chemical equation, which you need to know and memorize, looks like this:
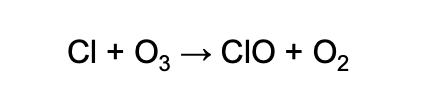
Next, if the ClO molecule finds a free oxygen atom, the oxygen atom will steal the O from the ClO to become O2, which releases the Cl atom back into the stratosphere to destroy more ozone.
The second equation looks like this:
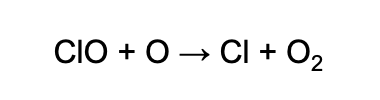
Now the Cl can repeat the process again, taking another ozone molecule and breaking it down as in the first equation.
A single CFC molecule can destroy 100,000 ozone molecules and it is thought that chlorine can remain in the stratosphere for 50 years or so.
UV light naturally breaks O3 down, and O3 is still formed naturally, but chlorine accelerates this process and puts the cycle out of balance.
One example of ozone depletion is the “hole” over Antarctica. This isn’t really a “hole” but rather very low levels of ozone over this area during the Antarctic spring.
However, the South Pole isn’t the only place with very low levels of ozone.
The result of all this chemistry is more UV light is able to get through the stratosphere and to the surface of the Earth.
UV light is dangerous because it damages the DNA in skin cells and leads to skin cancer, eye damage such as cataracts, and premature aging.
It can also suppress the immune system. In plants it can affect their growth and has been shown to cause problems for marine phytoplankton, the foundation of the marine food web.
Reducing Ozone Depletion
The main way we have tried to stop the problem of ozone depletion is to stop using ozone-depleting substances.
The Montreal Protocol (1987) and the Vienna Convention (1985) were treaties to control the release of these substances.
The main ozone-depleting substances are chlorofluorocarbons (CFCs), hydrochlorofluorocarbons, and halons.
Using things like hydrofluorocarbons can be a helpful replacement, but some are very potent greenhouse gases.
CFCs may also be reduced by inspecting and maintaining air conditioners and refrigeration appliances to prevent leaks.
The Greenhouse Effect
The greenhouse effect is shown in Figure 13.1. As you can see, solar radiation (infrared) from the sun is absorbed by Earth’s surface, warming the surface, and some is reflected back to space.
This is important because without the greenhouse effect, it would be too cold on Earth for life.
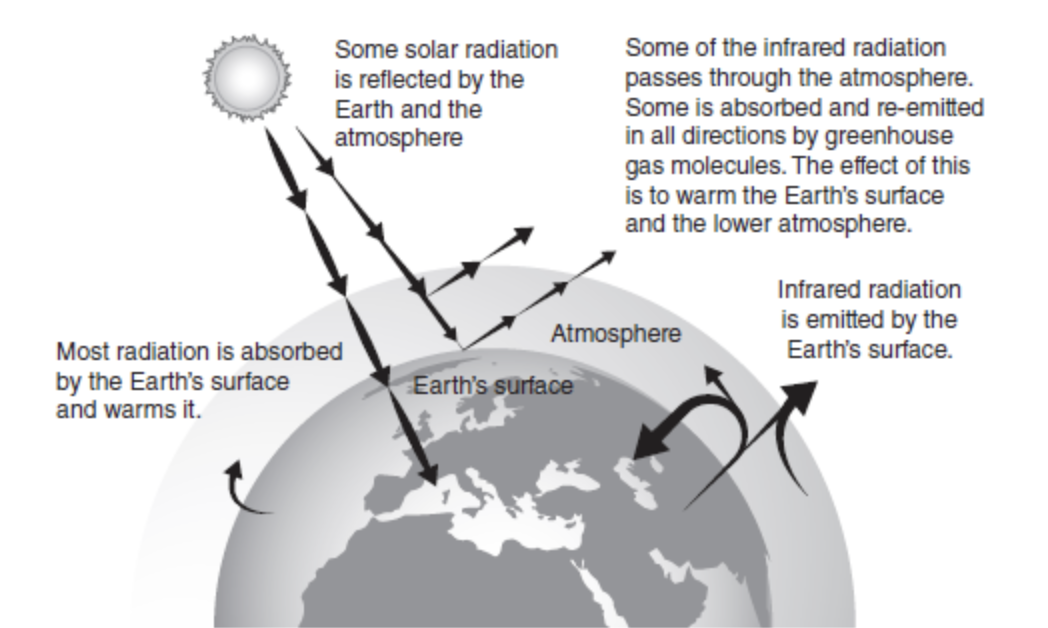
The main gases that are in our atmosphere that absorb the solar radiation are carbon dioxide, methane, water vapor, nitrous oxide, and chlorofluorocarbons.
Each of these has a different amount of global warming potential (GWP).
Water vapor’s GWP is unknown but not of great concern since the water cycle cycles relatively quickly.
GAS | SOURCES | GWP | ATMOSPHERIC LIFETIME |
|---|---|---|---|
Carbon dioxide | Decomposition, respiration, burning fossil fuels, deforestation | 1 | 50–200 years |
Methane | Burning of fossil fuels, livestock, landfills, decomposition, burning biomass, natural wetlands | 21 | 12 years |
Nitrous oxide | Soils, livestock manure, biomass or fossil fuel combustion, wastewater management | 310 | 120 years |
Chlorofluorocarbons | Refrigerants, aerosols, aircraft halons, solvents | 12,000–16,000 | 20–100 years |
Increases in the Greenhouse Gases
Some results of increased greenhouse gases that have impacted either the environment or harmed humans are:
Glaciers have shrunk.
Ice sheets are melting.
Plant and animal ranges have shifted.
Sea levels are rising.
Seas expand from thermal expansion.
Insects are moving to places that used to be too cold for them, and these insects can carry disease.
Droughts are threatening crops, wildlife, and freshwater supplies.
Biodiversity is affected as many species depend on ice for food and for a place to live.
Many organisms cannot move or adapt quickly enough to survive, so population numbers decrease, particularly specialist populations.
Global Climate Change
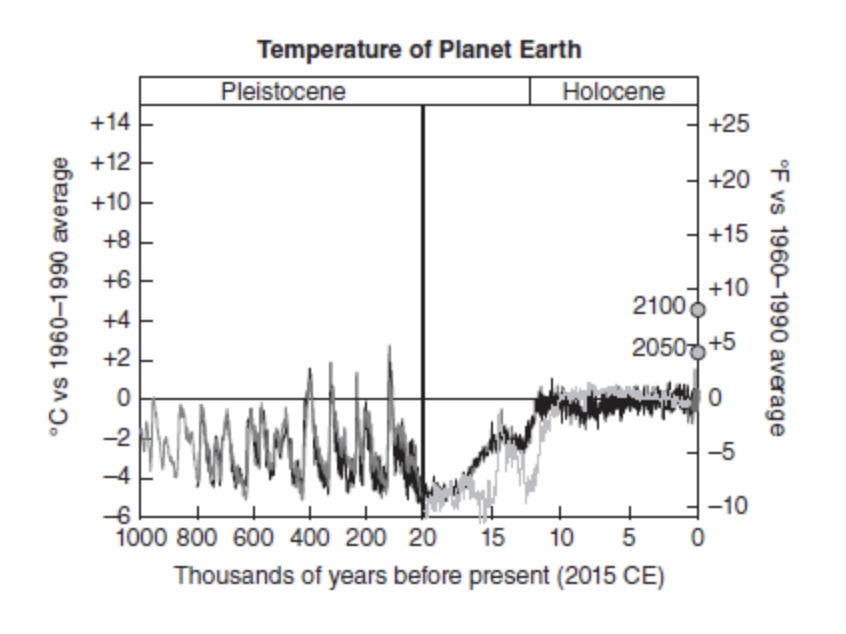
As you can see in Figure 13.2, Earth has had many different changes in temperature over its history.
There are times when it has been much warmer and times when it has been much colder.
Currently, we are in a warming trend, which as mentioned in the topic above, has led to many impacts on the environment and human health.
Sea levels are rising, which can flood coastal communities, impact ocean organisms that are now too deep to photosynthesize, and may create new habitats for other organisms.
Weather patterns are changing due to changes in the circulation of winds in the atmosphere.
Ocean currents, which push tropical heat toward the poles, are changing and weakening the oceans, global conveyor belt as warm freshwater melts into the sea, which stops the sinking of cold, salty water. This is changing climates across the globe.
Soil can be impacted if we have droughts and increased soil erosion from wind and water.
There is a positive feedback loop in the polar regions, where normally the ice reflects the sun’s rays back (albedo effect).
With a warmer planet we have more melting of the ice and less sunlight being reflected back to space.
This then increases the temperatures on Earth even more, causing more melting. A positive feedback loop is when one thing gets worse and worse, as in this example.
In addition, as ice and permafrost areas melt, methane is released; this a global warming gas mentioned above. This is another example of a positive feedback loop.
Ocean Warming
As our oceans get warmer because of climate change from greenhouse gases, there is a negative impact to ocean biodiversity.
For example, corals need zooxanthellae, an algae that lives in the tissues of coral and captures sunlight and converts this into energy for the coral animals.
When the temperature of the ocean is too warm, the zooxanthellae become under stress and can die or leave their coral host, causing the coral to die and turn white, a process known as coral bleaching.
A warmer ocean also can lead to loss of habitat to marine organisms.
Ocean Acidification
The ocean absorbs carbon dioxide from the atmosphere and the carbon dioxide reacts with the ocean water creating carbonic acid.
The oceans absorb almost a quarter of global CO2 emissions we create from burning fossil fuels and deforestation.
As the CO2 enters the ocean it causes the ocean to become more acidic (lower pH).
This acidity doesn’t allow animals like clams and mussels to make protective shells, due to the loss of calcium carbonate, and can impact corals since they also make protective shells.
Ocean acidification is happening because excess CO2 in the atmosphere is being absorbed by the ocean.
This excess carbon dioxide (CO2) reacts with water molecules (H2O) to form carbonic acid (H2CO3), resulting in more hydrogen ions and lowering the pH of the ocean.
The chemical formula looks like this:
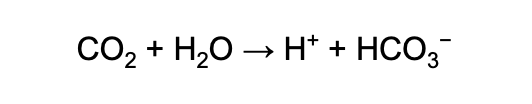
Invasive Species
An invasive species is one that is not native to an area.
Invasive species can harm the natural ecosystems by outcompeting with native species, reducing biodiversity, and altering habitats, sometimes leading to extinctions of native plants and animals.
Invasive species tend to be generalists and r-selected species, and they become invasive because of a lack of competition, no predators, and/or abundant resources.
The three main ways to control invasive species are:
Biological: Using natural enemies to control the pest species. This can work but sometimes the biological control backfires and the new species becomes a problem.
Mechanical: Mowing, hoeing, hand pulling, and more to control the pest species. This is expensive and time consuming.
Chemical: Using pesticides (such as herbicides, insecticides, and rodenticides). This can lead to the problems discussed earlier in this course on pesticides.
Many species are introduced to environments by human transport, either with the global trade of species (for example, exotic pets) or with humans traveling and intentionally or unintentionally bringing the species back with them.
Endangered Species
An endangered species is any animal or plant that is considered at risk of extinction.
A species can be listed as endangered at the international, national, or state level.
The Endangered Species Act has the endangered species listed on it and it is managed federally and allows the United States to enforce the international treaty known as CITES.
A species may become endangered for many reasons.
Habitat loss, invasive species, poaching, climate change, taking too many of a species in hunting and fishing, pollution, if the animal is highly specialized (lives in a certain place, eats a certain thing), competition within or with other species, and selective pressures on the species are all reasons animals and plants might become endangered.
If the species can’t adapt or move it might be in danger.
Humans can help prevent and can combat species becoming endangered by protecting the habitats where they live and protecting the species itself.
Human Impacts on Biodiversity
A way to remember how we harm biodiversity is the mnemonic device HIPPCO.
H—Habitat loss
I—Invasive species
P—Population growth
P—Pollution (but always name a chemical if you want to use this one—just “pollution” is too general)
C—Climate change, which leads to droughts, floods, sea level rising, and so on
O—Overharvesting (overfishing, overhunting)
Of all these reasons for loss of biodiversity, the greatest one is habitat loss. We harm habitats by cutting down forests, by building roads, by building human habitation in areas where there used to be large areas of land (habitat fragmentation), and so on.
We can prevent this loss of habitat by protecting large tracts of land so it can’t be developed, creating and enforcing legislation to protect biodiversity, preventing importation of nonnative species, creating habitat corridors so animals can move between native areas, preventing deforestation, using sustainable farming and ranching practices, and helping to restore areas that have been harmed by human or natural disasters.
Chapter 13: Global Change
Global climate change, ozone depletion, ocean warming, and endangered species are just a few of the ways humans have impacted the Earth.
You will need to look at global changes and be able to explain the causes and consequences of these. Make sure you can look at data and use this data to propose solutions or legislation to global problems.
One of the most important aspects that students confuse is the difference between global climate change and ozone depletion.
Local and regional human activities can have impacts at the global level.
The health of a species is closely tied to its ecosystem, and minor environmental changes can have a large impact.
Key Terms
Stratospheric ozone layer: The protected layer of our atmosphere that absorbs UV light.
Chlorofluorocarbons: A man-made chemical that works its way through the troposphere and into the stratosphere where it can destroy ozone molecules.
Ozone depletion: caused by chemicals like CFCs breaking down ozone in the stratosphere. This means less natural ozone to absorb UV light coming to Earth.
Greenhouse effect: The natural ability of Earth to absorb heat from the sun (infrared) and keep our planet warm enough to live on.
Global climate change: As more and more greenhouse gases are emitted into the atmosphere, we are trapping more heat from the sun and the temperature of the Earth is getting warmer. Sea levels are rising, estuaries are getting deeper, storms get stronger, insects are moving farther away from the equator, ice caps are melting, and permafrost is melting.
Positive feedback loop: When one thing causes something else to get progressively worse.
Coral bleaching: When the algae that lives in the coral animals dies or leaves the coral and causes the coral to die and turn white.
Ocean warming: When the Earth is getting warmer the oceans are also getting warmer. This is causing ice to melt, sea levels to rise, etc.
Ocean acidification: As more carbon dioxide is added to the atmosphere it gets into the ocean causing the ocean to have a lower pH and harming things like coral animals.
Invasive species: Nonnative species that are introduced to an environment and might outcompete the native species.
Endangered species: Any species that is threatened to go extinct.
Habitat fragmentation: When a habitat is split up into smaller areas with roads, homes, and more in the middle, it leads to loss of biodiversity.
Stratospheric Ozone Depletion
The stratospheric ozone layer is a shield around the Earth that absorbs most of the sun’s ultraviolet radiation (UV).
This layer of our atmosphere contains ozone (O3). It is the ozone (O3) and oxygen molecules (O2) in this layer that absorb the UV light.
The stratosphere is above the troposphere, which is the layer closest to the Earth’s surface; it extends from 10 km (6 miles) to about 50 km (31 miles) into space.
Because of the stratosphere’s ability to absorb the sun’s UV light, this dangerous radiation is prevented from passing to the Earth’s surface.
Without this stratospheric ozone layer life on Earth as we know it would not be possible.
Ozone is produced naturally in the stratosphere when the sun’s UV rays split O2 molecules into single oxygen atoms.
These oxygen atoms then combine with O2 to form O3 molecules, which are very good at absorbing UV light.
However, human-produced chemicals, such as chlorofluorocarbons (CFCs), although they are heavier than air, have been carried into the stratosphere by the air currents and mixing processes of the atmosphere.
These CFC molecules are hit by the sun’s UV energy and break up, releasing chlorine atoms.
The chlorine atoms then react with the O3 molecules, breaking up the O3 by taking one oxygen atom to form chlorine monoxide and O2.
The chemical equation, which you need to know and memorize, looks like this:

Next, if the ClO molecule finds a free oxygen atom, the oxygen atom will steal the O from the ClO to become O2, which releases the Cl atom back into the stratosphere to destroy more ozone.
The second equation looks like this:
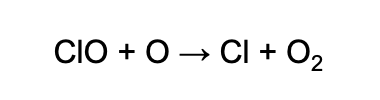
Now the Cl can repeat the process again, taking another ozone molecule and breaking it down as in the first equation.
A single CFC molecule can destroy 100,000 ozone molecules and it is thought that chlorine can remain in the stratosphere for 50 years or so.
UV light naturally breaks O3 down, and O3 is still formed naturally, but chlorine accelerates this process and puts the cycle out of balance.
One example of ozone depletion is the “hole” over Antarctica. This isn’t really a “hole” but rather very low levels of ozone over this area during the Antarctic spring.
However, the South Pole isn’t the only place with very low levels of ozone.
The result of all this chemistry is more UV light is able to get through the stratosphere and to the surface of the Earth.
UV light is dangerous because it damages the DNA in skin cells and leads to skin cancer, eye damage such as cataracts, and premature aging.
It can also suppress the immune system. In plants it can affect their growth and has been shown to cause problems for marine phytoplankton, the foundation of the marine food web.
Reducing Ozone Depletion
The main way we have tried to stop the problem of ozone depletion is to stop using ozone-depleting substances.
The Montreal Protocol (1987) and the Vienna Convention (1985) were treaties to control the release of these substances.
The main ozone-depleting substances are chlorofluorocarbons (CFCs), hydrochlorofluorocarbons, and halons.
Using things like hydrofluorocarbons can be a helpful replacement, but some are very potent greenhouse gases.
CFCs may also be reduced by inspecting and maintaining air conditioners and refrigeration appliances to prevent leaks.
The Greenhouse Effect
The greenhouse effect is shown in Figure 13.1. As you can see, solar radiation (infrared) from the sun is absorbed by Earth’s surface, warming the surface, and some is reflected back to space.
This is important because without the greenhouse effect, it would be too cold on Earth for life.

The main gases that are in our atmosphere that absorb the solar radiation are carbon dioxide, methane, water vapor, nitrous oxide, and chlorofluorocarbons.
Each of these has a different amount of global warming potential (GWP).
Water vapor’s GWP is unknown but not of great concern since the water cycle cycles relatively quickly.
GAS | SOURCES | GWP | ATMOSPHERIC LIFETIME |
|---|---|---|---|
Carbon dioxide | Decomposition, respiration, burning fossil fuels, deforestation | 1 | 50–200 years |
Methane | Burning of fossil fuels, livestock, landfills, decomposition, burning biomass, natural wetlands | 21 | 12 years |
Nitrous oxide | Soils, livestock manure, biomass or fossil fuel combustion, wastewater management | 310 | 120 years |
Chlorofluorocarbons | Refrigerants, aerosols, aircraft halons, solvents | 12,000–16,000 | 20–100 years |
Increases in the Greenhouse Gases
Some results of increased greenhouse gases that have impacted either the environment or harmed humans are:
Glaciers have shrunk.
Ice sheets are melting.
Plant and animal ranges have shifted.
Sea levels are rising.
Seas expand from thermal expansion.
Insects are moving to places that used to be too cold for them, and these insects can carry disease.
Droughts are threatening crops, wildlife, and freshwater supplies.
Biodiversity is affected as many species depend on ice for food and for a place to live.
Many organisms cannot move or adapt quickly enough to survive, so population numbers decrease, particularly specialist populations.
Global Climate Change
As you can see in Figure 13.2, Earth has had many different changes in temperature over its history.
There are times when it has been much warmer and times when it has been much colder.
Currently, we are in a warming trend, which as mentioned in the topic above, has led to many impacts on the environment and human health.

Sea levels are rising, which can flood coastal communities, impact ocean organisms that are now too deep to photosynthesize, and may create new habitats for other organisms.
Weather patterns are changing due to changes in the circulation of winds in the atmosphere.
Ocean currents, which push tropical heat toward the poles, are changing and weakening the oceans, global conveyor belt as warm freshwater melts into the sea, which stops the sinking of cold, salty water. This is changing climates across the globe.
Soil can be impacted if we have droughts and increased soil erosion from wind and water.
There is a positive feedback loop in the polar regions, where normally the ice reflects the sun’s rays back (albedo effect).
With a warmer planet we have more melting of the ice and less sunlight being reflected back to space.
This then increases the temperatures on Earth even more, causing more melting. A positive feedback loop is when one thing gets worse and worse, as in this example.
In addition, as ice and permafrost areas melt, methane is released; this a global warming gas mentioned above. This is another example of a positive feedback loop.
Ocean Warming
As our oceans get warmer because of climate change from greenhouse gases, there is a negative impact to ocean biodiversity.
For example, corals need zooxanthellae, an algae that lives in the tissues of coral and captures sunlight and converts this into energy for the coral animals.
When the temperature of the ocean is too warm, the zooxanthellae become under stress and can die or leave their coral host, causing the coral to die and turn white, a process known as coral bleaching.
A warmer ocean also can lead to loss of habitat to marine organisms.
Ocean Acidification
The ocean absorbs carbon dioxide from the atmosphere and the carbon dioxide reacts with the ocean water creating carbonic acid.
The oceans absorb almost a quarter of global CO2 emissions we create from burning fossil fuels and deforestation.
As the CO2 enters the ocean it causes the ocean to become more acidic (lower pH).
This acidity doesn’t allow animals like clams and mussels to make protective shells, due to the loss of calcium carbonate, and can impact corals since they also make protective shells.
Ocean acidification is happening because excess CO2 in the atmosphere is being absorbed by the ocean.
This excess carbon dioxide (CO2) reacts with water molecules (H2O) to form carbonic acid (H2CO3), resulting in more hydrogen ions and lowering the pH of the ocean.
The chemical formula looks like this:
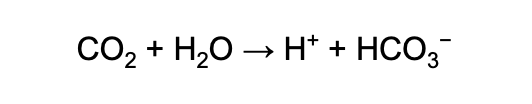
Invasive Species
An invasive species is one that is not native to an area.
Invasive species can harm the natural ecosystems by outcompeting with native species, reducing biodiversity, and altering habitats, sometimes leading to extinctions of native plants and animals.
Invasive species tend to be generalists and r-selected species, and they become invasive because of a lack of competition, no predators, and/or abundant resources.
The three main ways to control invasive species are:
Biological: Using natural enemies to control the pest species. This can work but sometimes the biological control backfires and the new species becomes a problem.
Mechanical: Mowing, hoeing, hand pulling, and more to control the pest species. This is expensive and time consuming.
Chemical: Using pesticides (such as herbicides, insecticides, and rodenticides). This can lead to the problems discussed earlier in this course on pesticides.
Many species are introduced to environments by human transport, either with the global trade of species (for example, exotic pets) or with humans traveling and intentionally or unintentionally bringing the species back with them.
Endangered Species
An endangered species is any animal or plant that is considered at risk of extinction.
A species can be listed as endangered at the international, national, or state level.
The Endangered Species Act has the endangered species listed on it and it is managed federally and allows the United States to enforce the international treaty known as CITES.
A species may become endangered for many reasons.
Habitat loss, invasive species, poaching, climate change, taking too many of a species in hunting and fishing, pollution, if the animal is highly specialized (lives in a certain place, eats a certain thing), competition within or with other species, and selective pressures on the species are all reasons animals and plants might become endangered.
If the species can’t adapt or move it might be in danger.
Humans can help prevent and can combat species becoming endangered by protecting the habitats where they live and protecting the species itself.
Human Impacts on Biodiversity
A way to remember how we harm biodiversity is the mnemonic device HIPPCO.
H—Habitat loss
I—Invasive species
P—Population growth
P—Pollution (but always name a chemical if you want to use this one—just “pollution” is too general)
C—Climate change, which leads to droughts, floods, sea level rising, and so on
O—Overharvesting (overfishing, overhunting)
Of all these reasons for loss of biodiversity, the greatest one is habitat loss. We harm habitats by cutting down forests, by building roads, by building human habitation in areas where there used to be large areas of land (habitat fragmentation), and so on.
We can prevent this loss of habitat by protecting large tracts of land so it can’t be developed, creating and enforcing legislation to protect biodiversity, preventing importation of nonnative species, creating habitat corridors so animals can move between native areas, preventing deforestation, using sustainable farming and ranching practices, and helping to restore areas that have been harmed by human or natural disasters.
 Knowt
Knowt