
Chapter 4: Carbon and the Molecular Diversity of Life
Carbon: The Backbone of Life
living organisms consists mostly of carbon-based compounds
carbon in unparalleled in its ability to form large, complex, and varied molecules
makes possible the diversity of organisms that have evolved on Earth
Proteins, DNA, carbohydrates, and other molecules that distinguish living matter are all composed of carbon compounds
Concept 4.1: Organic chemistry is the study of carbon compounds
organic chemistry: the study of compounds that contain carbon, regardless of origin
range from simple molecules to colossal ones, which as proteins
Organic Molecules and the Origin of Life on Earth
Stanley Miller’s classic experiment, trying to model how life first began, demonstrated the abiotic synthesis of organic compounds
experiments support the idea that abiotic synthesis of organic compounds, perhaps near volcanoes, could have been a stage in the origin of life
the overall percentages of the major elements of life — C, H, O, N, S, P — are suite uniform from one organism to another
because of carbon’s ability to form four bonds, these building blocks can be used to make an inexhaustible variety of organic molecules
the great diversity of organisms on the planet is due to the versatility of carbon
Concept 4.2: Carbon atoms can form diverse molecules by bonding to four other atoms
electron configuration is the key to an atom’s characteristics
electron configuration determines the kinds and number of bonds an atom will form with other atoms
we are carbon based, not silicon based because silicon holds onto other elements too strongly - can’t let go of elements easily when elements need to be used by the organism
The Formation of Bonds with Carbon
carbon has 4 valence electrons, so it can form four covalent bonds with a variety of atoms, making large complex molecules possible
in molecules with multiple carbons, each carbon bonded to four other atoms has a tetrahedral shape
when two carbon atoms are joined by a double bond, the atoms joined to the carbons are in the same plane as the carbons
double bonds don’t allow carbons to freely rotate (not dynamic like single bonds)
valence: the number of covalent bonds an atom can form
number of unpaired electrons in the valence shell of an atom is generally equal to its valence
the electron configuration of carbon gives it covalent compatibility with many different elements
the valence of carbons and its most frequent partners (hydrogen, oxygen, and nitrogen) are the building code for the architecture of living molecules
Molecular Diversity Arising from Variation in Carbon Skeletons
carbon chains form the skeletons of most organic molecules and they vary in length and shape
variation in carbon skeletons is an important source of the molecular complexity and diversity of living matter
Hydrocarbons
hydrocarbons: organic molecules consisting of only carbon and hydrogen
many organic molecules, such as fats, have hydrocarbon components
but, hydrocarbons are not prevalent in most living organisms
hydrocarbons can undergo reactions that release a large amount of energy
fat is more efficient to break down than sugar
Isomers
isomers: compounds with the same molecular formula but different structures and properties
structural isomers: have different covalent arrangements of their atoms
cis-trans isomers: have the same covalent bonds, but differ in their spatial arrangements
enantiomers : isomers that are mirror images of each other
cis-trans isomers require a double or triple bond and react differently in reactions
enantiomers are important in the pharmaceutical industry as two enantiomers of a drug may have different effects
usually only one isomer is biologically active
single bonded molecules will react the same, even if their bonds are rotated
double-bonded or triple-bonded molecules will react different in reactions if their bonds are rotated
Concept 4.3: A few chemical groups are key to molecular function
the properties of an organic molecule depend not only on the arrangement of its carbon skeleton but also on the various chemical groups attached to the skeleton
a number of characteristic groups can replace hydrogens attached to skeletons of organic molecules
The Chemical Groups Most Important in the Processes of Life
estradiol and testosterone are both steroids with a common carbon skeleton, in the form of four used rings
these sex hormones differ only in the chemical groups attached to the rings of the carbon skeleton
you would think estrogen and testosterone are a antagonist chemicals, but they’re actually pretty similar - only differ by groupings that they have
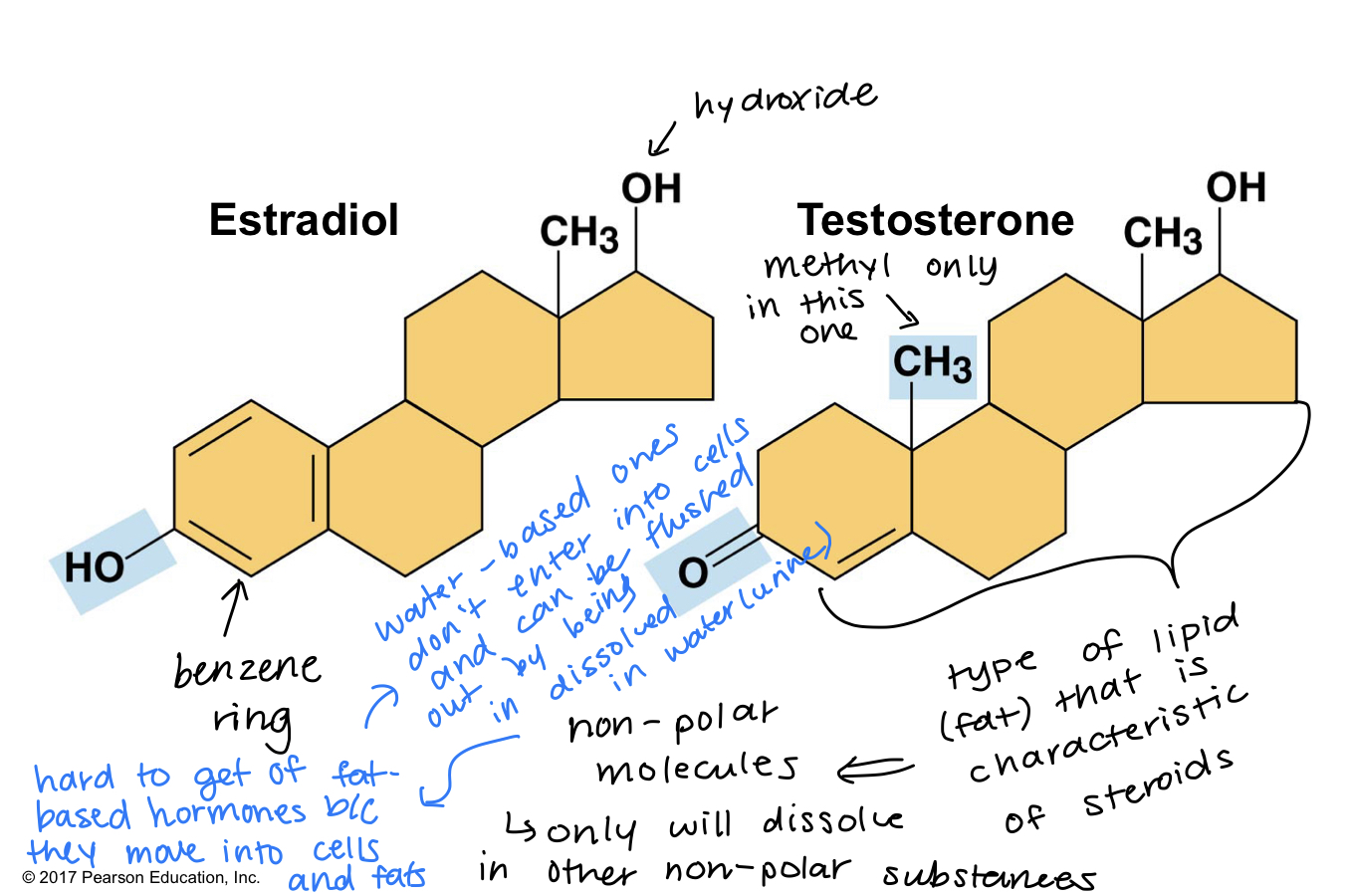
functional groups: the components of organic molecules that are most commonly involved in chemical reactions
the number and arrangement of functional groups gives each molecule its unique properties
ATP: An Important Source of Energy for Cellular Processes
Adenosine Triphosphate (ATP): an important organic phosphate that is the basis of life
ATP consists of an organic molecule called adenosine attached to a string of three phosphate groups
ATP is made in the inner-lining of the mitochondria
ATP is not a very stable molecule, allowing it to form ADP and release a phosphate group that then become inorganic
Chapter 4: Carbon and the Molecular Diversity of Life
Carbon: The Backbone of Life
living organisms consists mostly of carbon-based compounds
carbon in unparalleled in its ability to form large, complex, and varied molecules
makes possible the diversity of organisms that have evolved on Earth
Proteins, DNA, carbohydrates, and other molecules that distinguish living matter are all composed of carbon compounds
Concept 4.1: Organic chemistry is the study of carbon compounds
organic chemistry: the study of compounds that contain carbon, regardless of origin
range from simple molecules to colossal ones, which as proteins
Organic Molecules and the Origin of Life on Earth
Stanley Miller’s classic experiment, trying to model how life first began, demonstrated the abiotic synthesis of organic compounds
experiments support the idea that abiotic synthesis of organic compounds, perhaps near volcanoes, could have been a stage in the origin of life
the overall percentages of the major elements of life — C, H, O, N, S, P — are suite uniform from one organism to another
because of carbon’s ability to form four bonds, these building blocks can be used to make an inexhaustible variety of organic molecules
the great diversity of organisms on the planet is due to the versatility of carbon
Concept 4.2: Carbon atoms can form diverse molecules by bonding to four other atoms
electron configuration is the key to an atom’s characteristics
electron configuration determines the kinds and number of bonds an atom will form with other atoms
we are carbon based, not silicon based because silicon holds onto other elements too strongly - can’t let go of elements easily when elements need to be used by the organism
The Formation of Bonds with Carbon
carbon has 4 valence electrons, so it can form four covalent bonds with a variety of atoms, making large complex molecules possible
in molecules with multiple carbons, each carbon bonded to four other atoms has a tetrahedral shape
when two carbon atoms are joined by a double bond, the atoms joined to the carbons are in the same plane as the carbons
double bonds don’t allow carbons to freely rotate (not dynamic like single bonds)
valence: the number of covalent bonds an atom can form
number of unpaired electrons in the valence shell of an atom is generally equal to its valence
the electron configuration of carbon gives it covalent compatibility with many different elements
the valence of carbons and its most frequent partners (hydrogen, oxygen, and nitrogen) are the building code for the architecture of living molecules
Molecular Diversity Arising from Variation in Carbon Skeletons
carbon chains form the skeletons of most organic molecules and they vary in length and shape
variation in carbon skeletons is an important source of the molecular complexity and diversity of living matter
Hydrocarbons
hydrocarbons: organic molecules consisting of only carbon and hydrogen
many organic molecules, such as fats, have hydrocarbon components
but, hydrocarbons are not prevalent in most living organisms
hydrocarbons can undergo reactions that release a large amount of energy
fat is more efficient to break down than sugar
Isomers
isomers: compounds with the same molecular formula but different structures and properties
structural isomers: have different covalent arrangements of their atoms
cis-trans isomers: have the same covalent bonds, but differ in their spatial arrangements
enantiomers : isomers that are mirror images of each other
cis-trans isomers require a double or triple bond and react differently in reactions
enantiomers are important in the pharmaceutical industry as two enantiomers of a drug may have different effects
usually only one isomer is biologically active
single bonded molecules will react the same, even if their bonds are rotated
double-bonded or triple-bonded molecules will react different in reactions if their bonds are rotated
Concept 4.3: A few chemical groups are key to molecular function
the properties of an organic molecule depend not only on the arrangement of its carbon skeleton but also on the various chemical groups attached to the skeleton
a number of characteristic groups can replace hydrogens attached to skeletons of organic molecules
The Chemical Groups Most Important in the Processes of Life
estradiol and testosterone are both steroids with a common carbon skeleton, in the form of four used rings
these sex hormones differ only in the chemical groups attached to the rings of the carbon skeleton
you would think estrogen and testosterone are a antagonist chemicals, but they’re actually pretty similar - only differ by groupings that they have

functional groups: the components of organic molecules that are most commonly involved in chemical reactions
the number and arrangement of functional groups gives each molecule its unique properties
ATP: An Important Source of Energy for Cellular Processes
Adenosine Triphosphate (ATP): an important organic phosphate that is the basis of life
ATP consists of an organic molecule called adenosine attached to a string of three phosphate groups
ATP is made in the inner-lining of the mitochondria
ATP is not a very stable molecule, allowing it to form ADP and release a phosphate group that then become inorganic
 Knowt
Knowt