
Chapter 11: Introduction to Organic Chemistry: Hydrocarbons
11.1: Organic Compounds
Organic Chemistry: The study of carbon compounds.
Organic Compounds: Always contain carbon and hydrogen, and sometimes other nonmetals such as oxygen, sulfur, nitrogen, phosphorus, or a halogen.
Hydrocarbon: These organic compounds consist of only carbon and hydrogen.
A hydrocarbon is referred to as a saturated hydrocarbon when all the bonds in the molecule are single bonds.
11.2: Alkanes
Alkanes: These are a type of hydrocarbon in which the carbon atoms are connected only by single bonds
Names of alkanes end in –ane.
Methane, Ethane, Propane, Butane, etc.
IUPAC system: A system for naming organic compounds devised by the International Union of Pure and Applied Chemistry.
Alkanes with five or more carbon atoms in a chain are named using Greek prefixes:
pent, hex, hept, oct, non, and dec.
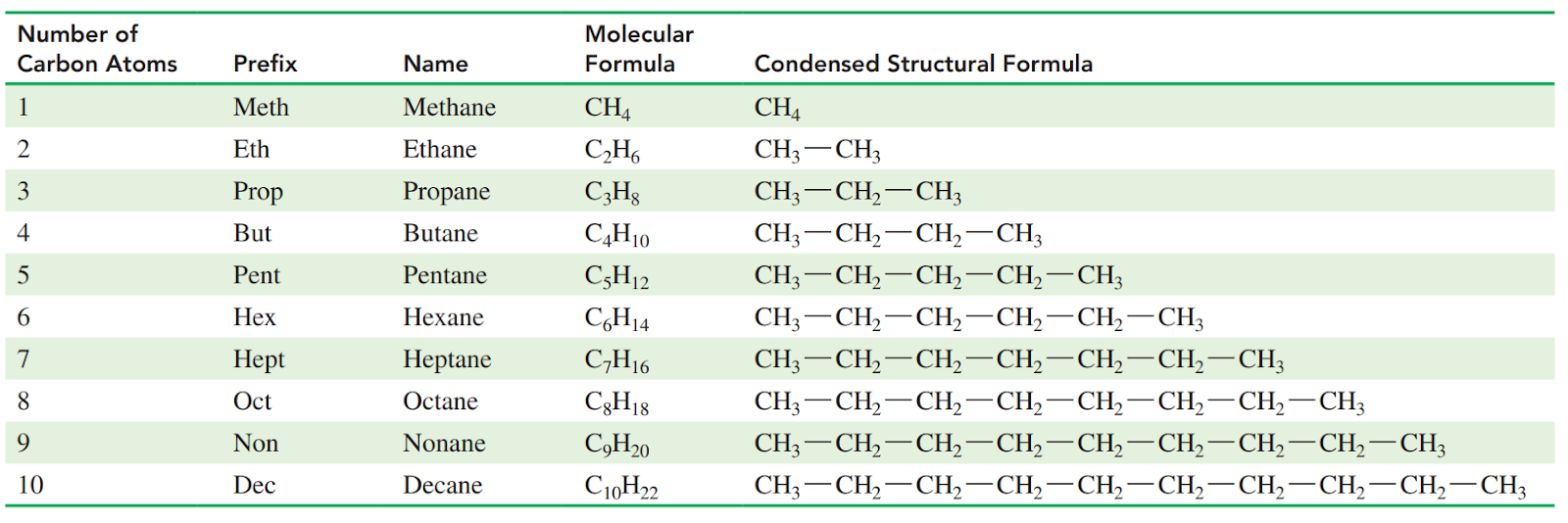
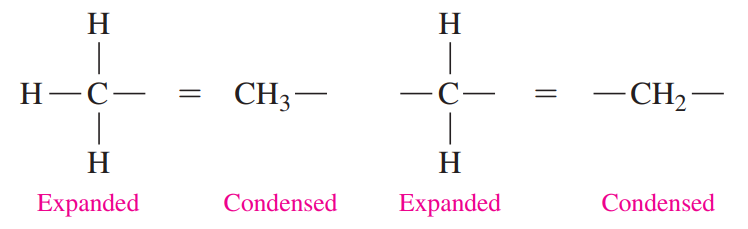
Condensed Structural Formulas
Each carbon atom and its attached hydrogen atoms are written as a group. A subscript indicates the number of hydrogen atoms bonded to each carbon atom.
Molecular formula: It gives the total number of carbon and hydrogen atoms, but does not indicate their arrangement in the molecule.
Skeletal Formula: A simplified structure that shows the carbon skeleton in which carbon atoms are represented at the end of each line or as corners.
Conformations of Alkanes
Because an alkane has only single carbon–carbon bonds, the groups attached to each C are not in fixed positions.
They can rotate freely about the bond connecting the carbon atoms. This motion is analogous to the independent rotation of the wheels of a toy car
Conformations: The different arrangements that occur during the rotation of a single bond.
Cycloalkanes: Hydrocarbons can also form cyclic or ring structures, which have two fewer hydrogen atoms than the corresponding alkanes.
Cyclopropane: The simplest cycloalkane that has a ring of three carbon atoms bonded to six hydrogen atoms.
11.3: Alkanes with Substituents
Substituent
When an alkane has four or more carbon atoms, the atoms can be arranged here, which is attached to the carbon chain.
Branched Alkane: An alkane with at least one branch.
Structural Isomers: When the two compounds have the same molecular formula but different arrangements of atoms.
Substituents in Alkanes
Alkyl Group: An alkane that is missing one hydrogen atom.
The alkyl group is named by replacing the –ane ending of the corresponding alkane name with –yl.
When a halogen atom is attached to a carbon chain, it is named as a halo group: fluoro, chloro, bromo, or iodo.
Haloalkanes: Halogen atoms replace hydrogen atoms in an alkane.
11.4: Properties of Alkanes
The first four alkanes — methane, ethane, propane, and butane — are gases at room temperature and are widely used as heating fuels.
Alkanes having five to eight carbon atoms are liquids at room temperature; they are highly volatile.
Liquid alkanes with 9 to 17 carbon atoms have higher boiling points and are found in kerosene, diesel, and jet fuels.
Motor oil: A mixture of high-molecular-weight liquid hydrocarbons and is used to lubricate the internal components of engines.
Mineral oil: A mixture of liquid hydrocarbons and is used as a laxative and a lubricant.
Alkanes with 18 or more carbon atoms are waxy solids at room temperature.
Paraffins: They are used in waxy coatings added to fruits and vegetables to retain moisture, inhibit mold, and enhance appearance.
Petroleum: A semisolid mixture of hydrocarbons with more than 25 carbon atoms used in ointments and cosmetics and as a lubricant.
Alkanes are nonpolar, which makes them insoluble in water.
However, they are soluble in nonpolar solvents such as other alkanes.
The carbon–carbon single bonds in alkanes are difficult to break, which makes them the least reactive family of organic compounds.
11.5: Alkenes and Alkynes
Alkenes and alkynes are families of hydrocarbons that contain double and triple bonds.
They are unsaturated hydrocarbons because they do not contain the maximum number of hydrogen atoms, as do alkanes.
They react with hydrogen gas to increase the number of hydrogen atoms to become alkanes, which are saturated hydrocarbons.
Alkenes: These contain one or more carbon–carbon double bonds that form when adjacent carbon atoms share two pairs of valence electrons.
Alkyne: A triple bond forms when two carbon atoms share three pairs of valence electrons.
The IUPAC names for alkenes and alkynes are similar to those of alkanes.
Using the alkane name with the same number of carbon atoms, the –ane ending is replaced with –ene for an alkene and –yne for an alkyne.
Cycloalkenes: These are some alkenes that have a double bond within a ring structure.
11.6: Cis–Trans Isomers
Cis–Trans Isomers: These are compounds that have different configurations because of the presence of a rigid structure in their molecule.
Cis Isomer: An isomer of an alkene in which similar groups in the double bond are on the same side.
Trans Isomer: An isomer of an alkene in which similar groups in the double bond are on opposite sides.
11.7: Additional Reactions
The most characteristic reaction of alkenes is the addition of atoms or groups of atoms to the carbon atoms in a double bond.
It occurs because double bonds are easily broken, providing electrons to form new single bonds.
Hydrogenation
H atoms add to each of the carbon atoms in a double bond of an alkene.
The double bonds are converted to single bonds in alkanes.
A catalyst is used to speed up the reaction.
Hydration
An alkene reacts with water (H — OH).
A hydrogen atom (H —) from water forms a bond with one carbon atom in the double bond, and the oxygen atom in —OH forms a bond with the other carbon.
The reaction is catalyzed by a strong acid such as H2SO4.
11.8: Aromatic Compounds
In 1825, Michael Faraday isolated a hydrocarbon called benzene, which had the molecular formula C6H6.
A molecule of benzene consists of a ring of six carbon atoms with one hydrogen atom attached to each carbon.
Aromatic Compounds: Family of benzene compounds.
In 1865, August Kekulé proposed that the carbon atoms in benzene were arranged in a flat ring with alternating single and double bonds between the carbon atoms.
Chapter 11: Introduction to Organic Chemistry: Hydrocarbons
11.1: Organic Compounds
Organic Chemistry: The study of carbon compounds.
Organic Compounds: Always contain carbon and hydrogen, and sometimes other nonmetals such as oxygen, sulfur, nitrogen, phosphorus, or a halogen.
Hydrocarbon: These organic compounds consist of only carbon and hydrogen.
A hydrocarbon is referred to as a saturated hydrocarbon when all the bonds in the molecule are single bonds.
11.2: Alkanes
Alkanes: These are a type of hydrocarbon in which the carbon atoms are connected only by single bonds
Names of alkanes end in –ane.
Methane, Ethane, Propane, Butane, etc.
IUPAC system: A system for naming organic compounds devised by the International Union of Pure and Applied Chemistry.
Alkanes with five or more carbon atoms in a chain are named using Greek prefixes:
pent, hex, hept, oct, non, and dec.

Condensed Structural Formulas
Each carbon atom and its attached hydrogen atoms are written as a group. A subscript indicates the number of hydrogen atoms bonded to each carbon atom.

Molecular formula: It gives the total number of carbon and hydrogen atoms, but does not indicate their arrangement in the molecule.
Skeletal Formula: A simplified structure that shows the carbon skeleton in which carbon atoms are represented at the end of each line or as corners.
Conformations of Alkanes
Because an alkane has only single carbon–carbon bonds, the groups attached to each C are not in fixed positions.
They can rotate freely about the bond connecting the carbon atoms. This motion is analogous to the independent rotation of the wheels of a toy car
Conformations: The different arrangements that occur during the rotation of a single bond.
Cycloalkanes: Hydrocarbons can also form cyclic or ring structures, which have two fewer hydrogen atoms than the corresponding alkanes.
Cyclopropane: The simplest cycloalkane that has a ring of three carbon atoms bonded to six hydrogen atoms.
11.3: Alkanes with Substituents
Substituent
When an alkane has four or more carbon atoms, the atoms can be arranged here, which is attached to the carbon chain.
Branched Alkane: An alkane with at least one branch.
Structural Isomers: When the two compounds have the same molecular formula but different arrangements of atoms.
Substituents in Alkanes
Alkyl Group: An alkane that is missing one hydrogen atom.
The alkyl group is named by replacing the –ane ending of the corresponding alkane name with –yl.
When a halogen atom is attached to a carbon chain, it is named as a halo group: fluoro, chloro, bromo, or iodo.
Haloalkanes: Halogen atoms replace hydrogen atoms in an alkane.
11.4: Properties of Alkanes
The first four alkanes — methane, ethane, propane, and butane — are gases at room temperature and are widely used as heating fuels.
Alkanes having five to eight carbon atoms are liquids at room temperature; they are highly volatile.
Liquid alkanes with 9 to 17 carbon atoms have higher boiling points and are found in kerosene, diesel, and jet fuels.
Motor oil: A mixture of high-molecular-weight liquid hydrocarbons and is used to lubricate the internal components of engines.
Mineral oil: A mixture of liquid hydrocarbons and is used as a laxative and a lubricant.
Alkanes with 18 or more carbon atoms are waxy solids at room temperature.
Paraffins: They are used in waxy coatings added to fruits and vegetables to retain moisture, inhibit mold, and enhance appearance.
Petroleum: A semisolid mixture of hydrocarbons with more than 25 carbon atoms used in ointments and cosmetics and as a lubricant.
Alkanes are nonpolar, which makes them insoluble in water.
However, they are soluble in nonpolar solvents such as other alkanes.
The carbon–carbon single bonds in alkanes are difficult to break, which makes them the least reactive family of organic compounds.
11.5: Alkenes and Alkynes
Alkenes and alkynes are families of hydrocarbons that contain double and triple bonds.
They are unsaturated hydrocarbons because they do not contain the maximum number of hydrogen atoms, as do alkanes.
They react with hydrogen gas to increase the number of hydrogen atoms to become alkanes, which are saturated hydrocarbons.
Alkenes: These contain one or more carbon–carbon double bonds that form when adjacent carbon atoms share two pairs of valence electrons.
Alkyne: A triple bond forms when two carbon atoms share three pairs of valence electrons.
The IUPAC names for alkenes and alkynes are similar to those of alkanes.
Using the alkane name with the same number of carbon atoms, the –ane ending is replaced with –ene for an alkene and –yne for an alkyne.
Cycloalkenes: These are some alkenes that have a double bond within a ring structure.
11.6: Cis–Trans Isomers
Cis–Trans Isomers: These are compounds that have different configurations because of the presence of a rigid structure in their molecule.
Cis Isomer: An isomer of an alkene in which similar groups in the double bond are on the same side.
Trans Isomer: An isomer of an alkene in which similar groups in the double bond are on opposite sides.
11.7: Additional Reactions
The most characteristic reaction of alkenes is the addition of atoms or groups of atoms to the carbon atoms in a double bond.
It occurs because double bonds are easily broken, providing electrons to form new single bonds.
Hydrogenation
H atoms add to each of the carbon atoms in a double bond of an alkene.
The double bonds are converted to single bonds in alkanes.
A catalyst is used to speed up the reaction.
Hydration
An alkene reacts with water (H — OH).
A hydrogen atom (H —) from water forms a bond with one carbon atom in the double bond, and the oxygen atom in —OH forms a bond with the other carbon.
The reaction is catalyzed by a strong acid such as H2SO4.
11.8: Aromatic Compounds
In 1825, Michael Faraday isolated a hydrocarbon called benzene, which had the molecular formula C6H6.
A molecule of benzene consists of a ring of six carbon atoms with one hydrogen atom attached to each carbon.
Aromatic Compounds: Family of benzene compounds.
In 1865, August Kekulé proposed that the carbon atoms in benzene were arranged in a flat ring with alternating single and double bonds between the carbon atoms.
 Knowt
Knowt
