
Light, Energy, and Electrons Chapter Test
Excited/Ground State
Ground State
Lowest energy state
This means that e- are found in shells closer to the nucleus
n =1
Excited State
Higher potential energy of an atom
n = 2 or higher
A form of heat, light, electrical, or mechanical energy is needed to go from the ground to an excited state
As electrons increase in energy, they move away from the nucleus and into outer shells
Absorption/Emission
Absorption (take in)
Energy moves electrons from a ground state to a higher energy state
Heat, light, electrical, chemical mechanical energy
Emission (give off)
Lets electrons fall back down to a lower energy state
Usually light
Energy must be absorbed for an electron to move to a higher state (one with a higher n value)
Energy is emitted when the electron moves to an orbit of lower energy (one with a lower n value)
The overall change in energy associated with "orbit jumping" is the difference in energy levels between the ending (final) and initial orbits
Wavelength/Frequency/Energy (ROY G BIV) (Both equations)
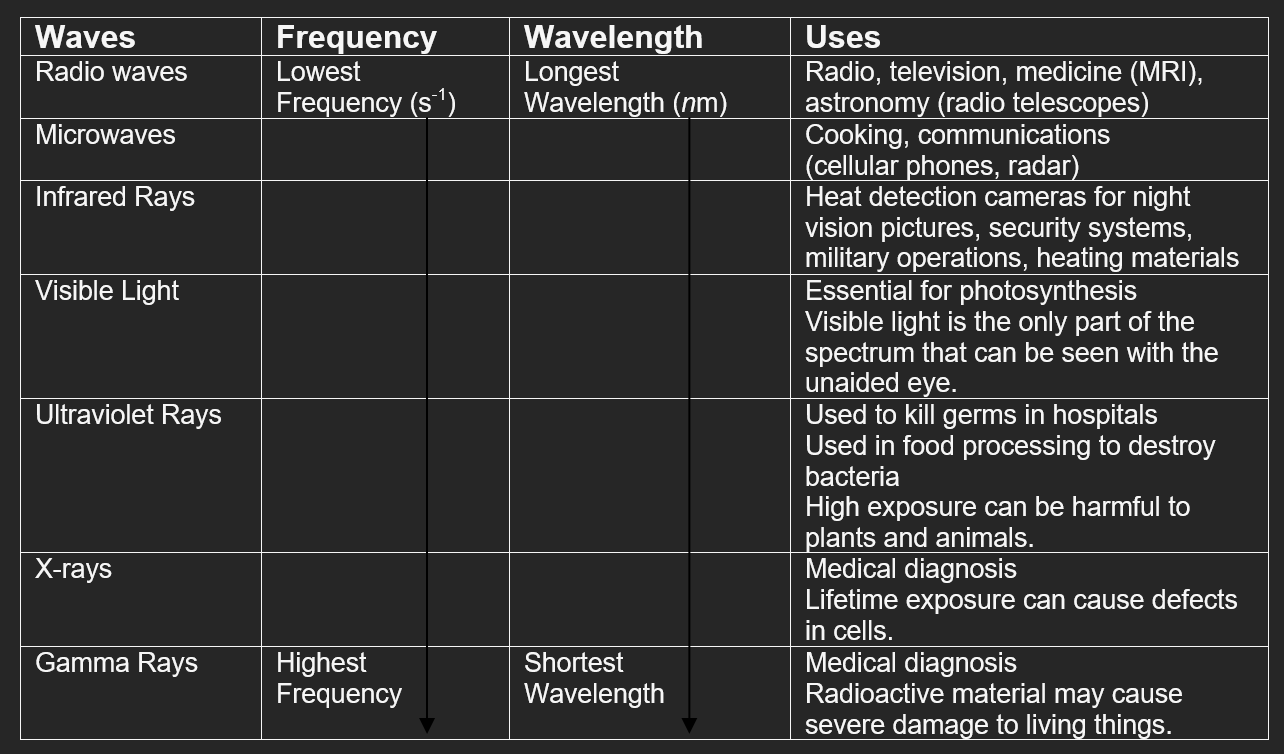
The wavelength (λ) of light is defined as the distance between the crests or troughs of a wave motion.
Wavelengths found in the electromagnetic spectrum (range of light) can be measured in units as large as 103 meters (radio waves) to 10-11 meters (gamma waves).
For the wavelengths of visible light (the light we see in color) the most common units used are nanometers (10-9 meters) and Angstroms (10-10 meters).
Frequency (ν) is the number of occurrences of a repeating event per unit time.
In the case of light, frequency refers to the number of times a wavelength is repeated per second. The unit used most often to describe frequency is Hz which means "per second" or /s.
The relationship between wavelength and frequency is related through the speed of light.
c = λν
c = 3.00 x 10^8 m/s
c is the speed of light
v is frequency
λ is wavelength
E=hv
h = 6.63x10^-34 J.s
E stands for energy (in Joules)
v stands for frequency [in reciprocal seconds – written s^-1 or Hertz (Hz)- 1Hz = 1 s^-1)
h is Planck’s constant.
If the frequency is known, it can easily be converted to wavelength using the speed of light and vice versa.
The wavelengths and frequencies of the light emitted by an atom (its emission spectrum) is determined by its electronic structure.
As each electron moves from a higher energy level (orbit) to a lower one, a different color is emitted.
Each shade of color has a unique wavelength based on the unique distance and energy.
As a wavelength increases in size, its frequency and energy (E) decrease.
As the frequency increases, the wavelength gets shorter.
As the frequency decreases, the wavelength gets longer.
Quantum Numbers (names and their meaning only)
Principle Quantum Number (n)
Indicates the main energy level (shell) occupied by the e- (distance from the nucleus)
Shell number (1st shell is closest to nucleus, 2nd is further, and so on)
Come from the Bohr Model
Values of n can only e positive integers (1, 2, 3, etc.)
As n increases, the orbital becomes larger; the electron has a higher energy and is farther away from the nucleus
Angular Momentum Quantum Number (l)
Indicates the general type of shapes of the orbitals
Nickname is subshell of n
Designated s, p, d, f
Values of l are zero and all positive integers less than equal to n-1
Magnetic Quantum Number (ml)
Indicates which exact orbital the electron is in
Describes the orientation of the orbital
Because an s orbital is spherical, it only has one orientation (ml = 0)
p orbitals can have three different orientations, one along the x-axis, one along the y-axis, and one along the z-axis
Spin Quantum Number (ms)
Indicates the two spin states of an e- in an orbital
Only 2 e- fit in each orbital, and they spin in opposite directions (up and down)
Possible m, values are -1/2, + 1/2
Spin is represented by dashes inside circles
Orbital notation
Shells
Distance from the nucleus (principle quantum number)
Represent ranges in energy
Subshells
Represent shapes (s, p, d, f)
One or more orbitals with the same set of n and l values
Each shell is divided into the number of subshells equal to the principal quantum number, n, for that shell.
The first shell consists of only the 1s subshell; the second shell consists of two subshells, 2s and 2p; the third of three subshell, 3s, 3p and 3d, and so forth.
Each subshell is divided into orbitals. Each s subshell consists of one orbital; each p subshell of three orbitals, each d subshell of five, and each f subshell of seven orbitals.
Angular momentum quantum number
Number of subshells in a shell
The number of subshells in a shell is equal to the shell number
1st shell - 1 subshell
2nd shell - 2 subshells
3rd shell - 3 subshells
Electron Filling Order: 1s 2s 2p…
Electron filling tree
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f
Also known as the Aufbau principle
Orbitals
Three dimensional space that electrons most probably occupy
Defined by n, l, and ml
The math equation treats electrons like waves
You can solve the equation to get the shape in space in which electrons are
Shapes look like “clouds” of probability
Number of orbitals per subshell: S P D F/ Number of electrons per orbital and per subshell
S subshell
Spherical shaped
1 orbital, 2 e-
P subshell
Peanut shaped
3 orbitals, 6 e-
D subshell
Double peanut shaped
5 orbitals, 10 e-
F subshell
Flower shaped
7 orbitals, 14 e-
Each subshell’s name comes from the old spectroscopic description of the lines corresponding to these orbitals
1st subshell in a shell = s subshell → sharp
2nd subshell in a shell = p subshell → principal
3rd subshell in a shell = d subshell → diffuse
4th subshell in a shell = f subshell → fundamental
Aufbau Principle
“Building up”
An electron occupies that lowest energy possible
The levels follow a pattern of increasing energy
Fill starting at nucleus (Bohr Models)
P subshell → 3 orbitals
Fill left to right
Pauli Exclusion Principle
No 2 electrons have the same spin if they are in the same orbital
Hund’s Rule
Electrons do not pair up until there are no more empty orbitals in that subshell
Orbital Notation
Representation of electron configuration in which orbital is represented by a circle and dashes
Each dash represents the number of electrons in each subshell
Electron Configuration
The correct order electrons are filled in
The most stable, or ground, electron configuration of an atom is that in which the electrons are in the lowest possible energy level
All subshells contain a certain number of orbitals
May be occupied by a single e- or by 2e- having opposite spins
Like cups
Shells don’t always get filled from 1 to 2 to 3 etc. because some subshells overlap
Valence/Core electrons
Valence = outermost
Valence electrons are electrons in the outer shells
Core electrons are electrons in the inner shells
Count the total electrons in the highest shell number
Do not count electrons in d subshells
Do count s and p
The Periodic Table and ordering of electrons
Rows (periods)
All of the elements in the row have the same number of orbitals
Columns (groups)
All of the elements in the column have the same number of (valence) electrons
Share similar chemical and physical properties because they possess the same # of valence electrons

Light, Energy, and Electrons Chapter Test
Excited/Ground State
Ground State
Lowest energy state
This means that e- are found in shells closer to the nucleus
n =1
Excited State
Higher potential energy of an atom
n = 2 or higher
A form of heat, light, electrical, or mechanical energy is needed to go from the ground to an excited state
As electrons increase in energy, they move away from the nucleus and into outer shells
Absorption/Emission
Absorption (take in)
Energy moves electrons from a ground state to a higher energy state
Heat, light, electrical, chemical mechanical energy
Emission (give off)
Lets electrons fall back down to a lower energy state
Usually light
Energy must be absorbed for an electron to move to a higher state (one with a higher n value)
Energy is emitted when the electron moves to an orbit of lower energy (one with a lower n value)
The overall change in energy associated with "orbit jumping" is the difference in energy levels between the ending (final) and initial orbits
Wavelength/Frequency/Energy (ROY G BIV) (Both equations)
The wavelength (λ) of light is defined as the distance between the crests or troughs of a wave motion.
Wavelengths found in the electromagnetic spectrum (range of light) can be measured in units as large as 103 meters (radio waves) to 10-11 meters (gamma waves).
For the wavelengths of visible light (the light we see in color) the most common units used are nanometers (10-9 meters) and Angstroms (10-10 meters).
Frequency (ν) is the number of occurrences of a repeating event per unit time.
In the case of light, frequency refers to the number of times a wavelength is repeated per second. The unit used most often to describe frequency is Hz which means "per second" or /s.
The relationship between wavelength and frequency is related through the speed of light.
c = λν
c = 3.00 x 10^8 m/s
c is the speed of light
v is frequency
λ is wavelength
E=hv
h = 6.63x10^-34 J.s
E stands for energy (in Joules)
v stands for frequency [in reciprocal seconds – written s^-1 or Hertz (Hz)- 1Hz = 1 s^-1)
h is Planck’s constant.
If the frequency is known, it can easily be converted to wavelength using the speed of light and vice versa.
The wavelengths and frequencies of the light emitted by an atom (its emission spectrum) is determined by its electronic structure.
As each electron moves from a higher energy level (orbit) to a lower one, a different color is emitted.
Each shade of color has a unique wavelength based on the unique distance and energy.
As a wavelength increases in size, its frequency and energy (E) decrease.
As the frequency increases, the wavelength gets shorter.
As the frequency decreases, the wavelength gets longer.

Quantum Numbers (names and their meaning only)
Principle Quantum Number (n)
Indicates the main energy level (shell) occupied by the e- (distance from the nucleus)
Shell number (1st shell is closest to nucleus, 2nd is further, and so on)
Come from the Bohr Model
Values of n can only e positive integers (1, 2, 3, etc.)
As n increases, the orbital becomes larger; the electron has a higher energy and is farther away from the nucleus
Angular Momentum Quantum Number (l)
Indicates the general type of shapes of the orbitals
Nickname is subshell of n
Designated s, p, d, f
Values of l are zero and all positive integers less than equal to n-1
Magnetic Quantum Number (ml)
Indicates which exact orbital the electron is in
Describes the orientation of the orbital
Because an s orbital is spherical, it only has one orientation (ml = 0)
p orbitals can have three different orientations, one along the x-axis, one along the y-axis, and one along the z-axis
Spin Quantum Number (ms)
Indicates the two spin states of an e- in an orbital
Only 2 e- fit in each orbital, and they spin in opposite directions (up and down)
Possible m, values are -1/2, + 1/2
Spin is represented by dashes inside circles
Orbital notation
Shells
Distance from the nucleus (principle quantum number)
Represent ranges in energy
Subshells
Represent shapes (s, p, d, f)
One or more orbitals with the same set of n and l values
Each shell is divided into the number of subshells equal to the principal quantum number, n, for that shell.
The first shell consists of only the 1s subshell; the second shell consists of two subshells, 2s and 2p; the third of three subshell, 3s, 3p and 3d, and so forth.
Each subshell is divided into orbitals. Each s subshell consists of one orbital; each p subshell of three orbitals, each d subshell of five, and each f subshell of seven orbitals.
Angular momentum quantum number
Number of subshells in a shell
The number of subshells in a shell is equal to the shell number
1st shell - 1 subshell
2nd shell - 2 subshells
3rd shell - 3 subshells
Electron Filling Order: 1s 2s 2p…
Electron filling tree
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f
Also known as the Aufbau principle
Orbitals
Three dimensional space that electrons most probably occupy
Defined by n, l, and ml
The math equation treats electrons like waves
You can solve the equation to get the shape in space in which electrons are
Shapes look like “clouds” of probability
Number of orbitals per subshell: S P D F/ Number of electrons per orbital and per subshell
S subshell
Spherical shaped
1 orbital, 2 e-
P subshell
Peanut shaped
3 orbitals, 6 e-
D subshell
Double peanut shaped
5 orbitals, 10 e-
F subshell
Flower shaped
7 orbitals, 14 e-
Each subshell’s name comes from the old spectroscopic description of the lines corresponding to these orbitals
1st subshell in a shell = s subshell → sharp
2nd subshell in a shell = p subshell → principal
3rd subshell in a shell = d subshell → diffuse
4th subshell in a shell = f subshell → fundamental
Aufbau Principle
“Building up”
An electron occupies that lowest energy possible
The levels follow a pattern of increasing energy
Fill starting at nucleus (Bohr Models)
P subshell → 3 orbitals
Fill left to right
Pauli Exclusion Principle
No 2 electrons have the same spin if they are in the same orbital
Hund’s Rule
Electrons do not pair up until there are no more empty orbitals in that subshell
Orbital Notation
Representation of electron configuration in which orbital is represented by a circle and dashes
Each dash represents the number of electrons in each subshell
Electron Configuration
The correct order electrons are filled in
The most stable, or ground, electron configuration of an atom is that in which the electrons are in the lowest possible energy level
All subshells contain a certain number of orbitals
May be occupied by a single e- or by 2e- having opposite spins
Like cups
Shells don’t always get filled from 1 to 2 to 3 etc. because some subshells overlap
Valence/Core electrons
Valence = outermost
Valence electrons are electrons in the outer shells
Core electrons are electrons in the inner shells
Count the total electrons in the highest shell number
Do not count electrons in d subshells
Do count s and p
The Periodic Table and ordering of electrons
Rows (periods)
All of the elements in the row have the same number of orbitals
Columns (groups)
All of the elements in the column have the same number of (valence) electrons
Share similar chemical and physical properties because they possess the same # of valence electrons

 Knowt
Knowt
