
Chapter 1: Atoms, Elements, Compounds and Chemical Equations
1.1-Atoms
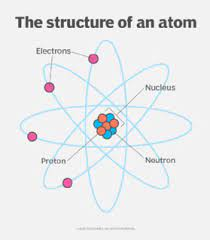
Atoms contain protons, neutrons and electrons
Atoms have a radius of about 10 nanometres(1x10(-9)). There are a few different modern models of the atom.
The nucleus
It’s in the middle of the atom
It contains protons and neutrons
The nucleus has a radius of around 1x10(-14) which is around 1/10,000 the radius of an atom
It has a positive charge because of the protons
Almost the whole mass of the atom is concentrated in the nucleus
The electrons
Move around the nucleus in electron shells
They’re negatively charges and tiny, but they cover a lot of space
Electrons have virtually no mass
Particles, relative mass and charge
Proton:1 and +1
Neutron:1 and 0
Electron:Very small and -1
Number of protons equals number of electrons
Atoms are neutral-they have no charge overall(unlike ions).
This is because they have the same number of protons as electrons
The charge on the electrons is the same size as the charge on the protons, but opposite-so the charges cancel out
In an ion, the number of protons doesn’t equal the number of electrons.
This means it has an overall charge. For example, an ion with a 2- charge, has two more electrons than protons
Atomic Numbers and Mass Number describe an Atom
The nuclear symbol of an atom tells you its atomic(proton) number and mass number
The atomic number tells you how many protons there are
The mass number tells you the total number of protons and neutrons in the atom
To get the number of neutrons, just subtract the atomic number from the mass number
1.2-Elements
An element is a substance made up of atoms that all have the same number of protons in their nucleus
Elements consist of atoms with the same atomic number
Atoms can have different numbers of protons, neutrons and electrons.
It’s the number of protons in the nucleus that decides what type of atom it is
For example, an atom with one proton in its nucleus is hydrogen and an atom with two protons is helium.
There are about 100 different elements
So all the atoms of a particular element have the same number of protons and different elements have atoms with different numbers of protons
Atoms can be represented by symbols
Atoms of each element can be represented by a one or two letter symbol-it’s a type of shorthand that saves you the bother of having to write the full name of the element.
e.g. C=carbon, O-oxygen, Mg=magnesium
or e.g. Na=sodium, Fe=iron, Pb-lead
Isotopes are the same except for extra neutrons
Isotopes are different forms of the same element, which have the same number of protons but a different number of neutrons
So isotopes have the same atomic number but different mass numbers
A very popular example of a pair of isotopes are carbon 12 and carbon 13
Carbon 12:
6 protons
6 electrons
6 neutrons
Carbon 13
6 protons
6 electrons
7 neutrons
Because many elements can exist as a number of different isotopes, relative atomic mass is used instead of mass number when referring to the element as a whole.
This is an average mass taking into account the different masses and abundances of all the isotopes that make up the element
You can use a formula to work out the relative atomic mass:
Relative atomic mass = sum of(isotopes abundance x isotope mass number) / sum of abundance of all the isotopes
1.3-Compounds
Atoms can join together to make compounds
When elements react, atoms combine with other atoms to form compounds
Compounds: substances formed form two or more elements
The atoms of each are in fixed properties throughout the compound and they’re held together by chemical bounds
Making bonds involves atoms giving away, taking or sharing electrons.
Only the electrons are involved-the nuclei of the atoms aren’t affected at all when a bond is made
It’s usually difficult to separate the original element of a compound out again
A chemical reaction is needed to do this
A compound which is formed from a metal and a non-metal consists of ions.
The metal atoms lose electrons to form positive ions and the non-metal atoms gain electrons to form negative ions.
The opposite charges(positive and negative) of the ions mean that they’re strongly attracted to each other.
This is called ionic bonding.
Examples of compounds which are bonded ionically include sodium chloride, magnesium oxide and calcium oxide
A compound formed from non-metals consists of molecules.
Each other shares an electron with another atom-this is called covalent bonding.
Examples of compounds that are bonded covalently include hydrogen chloride gas. carbon monoxide and water
The properties of a compound are usually totally different from the properties of the original elements.
For example, if iron and sulfur react, the compound formed is a dull grey solid lump, and doesn’t behave anything like either iron or sulfur
A formula shows what atoms are in a compound
Just as elements can be represented by symbols, compounds can be represented by formulas.
The formulas are made up of elemental symbols in the same proportions that the elements can be found in the compound
1.4-Chemical Equations
Chemical changes are shown using chemical equations
One way to show a chemical reaction is to write a word equation.
It’s not as quick as using chemical symbols and you can’t tell straight away what’s happened to each of the atoms, but it’s dead easy.
Here’s an example-you’re told that methane burns in oxygen giving carbon dioxide and water
Methane + Oxygen - Carbon Dioxide + Water
The molecules on the left-hand side of the equation are called reactants(because they react with each other)
The molecules on the right-hand side are called the products(because they’ve been produced from the reactants
Symbol equations show the atoms on both sides
Chemical changes can be shown in a kind of shorthand using symbol equations.
Symbol equations just show the symbols or formulas of the reactants and products
Magnesium + Oxygen - Magnesium Oxide
2Mg + 02 - 2Mg0
Symbol equations need to be balanced
There must always be the same number of atoms on both sides-they can’t just disappear
You balance the equation by putting numbers in front of the formulas where needed.
Take this equation for reacting sulfuric acid with sodium hydroxide:
H2S04 + Na0H - Na2S04 + H20
The formulas are all correct but the numbers of some atoms don’t match up on both sides
You can’t change formulas like H2SO4 to H2SO5. You can only put numbers in front of them.
The more you practise, the quicker you get, but all you do is this:
Find an element that doesn’t balance and pencil in a number to try and sort it out
See where it gets you. It may create another imbalance, but if so, pencil in another number and see where that gets you
Carry on chasing unbalanced elements and it’ll sort itself out pretty quickly
Chapter 1: Atoms, Elements, Compounds and Chemical Equations
1.1-Atoms
Atoms contain protons, neutrons and electrons
Atoms have a radius of about 10 nanometres(1x10(-9)). There are a few different modern models of the atom.
The nucleus
It’s in the middle of the atom
It contains protons and neutrons
The nucleus has a radius of around 1x10(-14) which is around 1/10,000 the radius of an atom
It has a positive charge because of the protons
Almost the whole mass of the atom is concentrated in the nucleus
The electrons
Move around the nucleus in electron shells
They’re negatively charges and tiny, but they cover a lot of space
Electrons have virtually no mass

Particles, relative mass and charge
Proton:1 and +1
Neutron:1 and 0
Electron:Very small and -1
Number of protons equals number of electrons
Atoms are neutral-they have no charge overall(unlike ions).
This is because they have the same number of protons as electrons
The charge on the electrons is the same size as the charge on the protons, but opposite-so the charges cancel out
In an ion, the number of protons doesn’t equal the number of electrons.
This means it has an overall charge. For example, an ion with a 2- charge, has two more electrons than protons
Atomic Numbers and Mass Number describe an Atom
The nuclear symbol of an atom tells you its atomic(proton) number and mass number
The atomic number tells you how many protons there are
The mass number tells you the total number of protons and neutrons in the atom
To get the number of neutrons, just subtract the atomic number from the mass number

1.2-Elements
An element is a substance made up of atoms that all have the same number of protons in their nucleus
Elements consist of atoms with the same atomic number
Atoms can have different numbers of protons, neutrons and electrons.
It’s the number of protons in the nucleus that decides what type of atom it is
For example, an atom with one proton in its nucleus is hydrogen and an atom with two protons is helium.
There are about 100 different elements
So all the atoms of a particular element have the same number of protons and different elements have atoms with different numbers of protons
Atoms can be represented by symbols
Atoms of each element can be represented by a one or two letter symbol-it’s a type of shorthand that saves you the bother of having to write the full name of the element.
e.g. C=carbon, O-oxygen, Mg=magnesium
or e.g. Na=sodium, Fe=iron, Pb-lead
Isotopes are the same except for extra neutrons
Isotopes are different forms of the same element, which have the same number of protons but a different number of neutrons
So isotopes have the same atomic number but different mass numbers
A very popular example of a pair of isotopes are carbon 12 and carbon 13
Carbon 12:
6 protons
6 electrons
6 neutrons
Carbon 13
6 protons
6 electrons
7 neutrons
Because many elements can exist as a number of different isotopes, relative atomic mass is used instead of mass number when referring to the element as a whole.
This is an average mass taking into account the different masses and abundances of all the isotopes that make up the element
You can use a formula to work out the relative atomic mass:
Relative atomic mass = sum of(isotopes abundance x isotope mass number) / sum of abundance of all the isotopes
1.3-Compounds
Atoms can join together to make compounds
When elements react, atoms combine with other atoms to form compounds
Compounds: substances formed form two or more elements
The atoms of each are in fixed properties throughout the compound and they’re held together by chemical bounds
Making bonds involves atoms giving away, taking or sharing electrons.
Only the electrons are involved-the nuclei of the atoms aren’t affected at all when a bond is made
It’s usually difficult to separate the original element of a compound out again
A chemical reaction is needed to do this
A compound which is formed from a metal and a non-metal consists of ions.
The metal atoms lose electrons to form positive ions and the non-metal atoms gain electrons to form negative ions.
The opposite charges(positive and negative) of the ions mean that they’re strongly attracted to each other.
This is called ionic bonding.
Examples of compounds which are bonded ionically include sodium chloride, magnesium oxide and calcium oxide
A compound formed from non-metals consists of molecules.
Each other shares an electron with another atom-this is called covalent bonding.
Examples of compounds that are bonded covalently include hydrogen chloride gas. carbon monoxide and water
The properties of a compound are usually totally different from the properties of the original elements.
For example, if iron and sulfur react, the compound formed is a dull grey solid lump, and doesn’t behave anything like either iron or sulfur
A formula shows what atoms are in a compound
Just as elements can be represented by symbols, compounds can be represented by formulas.
The formulas are made up of elemental symbols in the same proportions that the elements can be found in the compound
1.4-Chemical Equations
Chemical changes are shown using chemical equations
One way to show a chemical reaction is to write a word equation.
It’s not as quick as using chemical symbols and you can’t tell straight away what’s happened to each of the atoms, but it’s dead easy.
Here’s an example-you’re told that methane burns in oxygen giving carbon dioxide and water
Methane + Oxygen - Carbon Dioxide + Water
The molecules on the left-hand side of the equation are called reactants(because they react with each other)
The molecules on the right-hand side are called the products(because they’ve been produced from the reactants
Symbol equations show the atoms on both sides
Chemical changes can be shown in a kind of shorthand using symbol equations.
Symbol equations just show the symbols or formulas of the reactants and products
Magnesium + Oxygen - Magnesium Oxide
2Mg + 02 - 2Mg0
Symbol equations need to be balanced
There must always be the same number of atoms on both sides-they can’t just disappear
You balance the equation by putting numbers in front of the formulas where needed.
Take this equation for reacting sulfuric acid with sodium hydroxide:
H2S04 + Na0H - Na2S04 + H20
The formulas are all correct but the numbers of some atoms don’t match up on both sides
You can’t change formulas like H2SO4 to H2SO5. You can only put numbers in front of them.
The more you practise, the quicker you get, but all you do is this:
Find an element that doesn’t balance and pencil in a number to try and sort it out
See where it gets you. It may create another imbalance, but if so, pencil in another number and see where that gets you
Carry on chasing unbalanced elements and it’ll sort itself out pretty quickly
 Knowt
Knowt
