
AP Environmental Science 9.1-9.2 - Ozone Depletion
What is the Ozone Shield?
Ozone Shield: a natural process that filters ultraviolet (UV) radiation before it reaches the lower atmosphere.
The layer of ozone gas (O3) in the upper stratosphere that screens out harmful ultraviolet radiation from the sun.
If the full amount of ultraviolet radiation falling on the stratosphere reached Earth’s surface; it is doubtful that any life could survive.
Ozone Depletion
How does Ozone Depletion Occur?
Overview
CFCs (clorofluorocarbons) emitted into atmosphere; they’re stable, move from troposphere to stratosphere
UV light breaks off chlorine molecule (Cl) from the CFC particle
Cl acts as a catalyst to break down ozone (O3)
catalyst – promotes a chemical reaction without itself being used up in the reaction
Shifts equilibrium of oxygen / ozone reaction
Summary of Reactions
Cl₃CF (example CFC) + UV → Cl+CCl₂
Cl+O₃ → ClO + O₂
ClO + O → Cl + O₂
This cycle repeats itself several times
“From Dream Chemicals to Nightmare Chemicals”
Thomas Midgley, Jr. A General Motors chemist, discovered the first chlorofluorocarbon (CFC) in 1930.
Family of highly useful CFCs – trichlorofluoromethane and dichlorodifluoromethane (AKA; freons)
Stable, odorless, nonflammable, nontoxic, and noncorrosive
Used in air conditioners, refrigerators, aerosol spray cans, cleaners for electronic parts, sterilants for hospital instruments, fumigants for granaries, bubbles in plastic foam used for packaging.
Consequences of Ozone Depletion
Humans
Increase in skin cancer & cataracts, especially in the southern hemisphere
More ozone near earth’s surface, produced in photochemical smog – lung problems, suppressed immune response, cancer
Threat of Ozone Depletion
Radiation from the sun includes ultraviolet (UV) radiation; UVA and UVB
UV radiation penetrates the atmosphere and is absorbed by biological tissues damaging protein and DNA molecules at the surfaces of all living things (sunburn).
Most of the dangerous UVB radiation (over 99%) is absorbed by ozone in the stratosphere.
Other Organisms
Primary Producers:
Reduction in phytoplankton
Lower crop yields
Decline in forest productivity
Animals:
Species disruption through increased exposure to UV-B radiation
Disruption of food chain
Effect of Ozone Depletion
Ozone Depleting Chemicals
Chlorofluorocarbons (CFCs)
Halons: fire extinguishers
Methyl bromide: fumigant
Carbon tetrachloride: cheap, highly toxic solvent
Methyl chloroform: cleaning solvent-clothes & metals
Hydrogen chloride; U.S. space shuttles
Ozone Hole
Seasonal thinning of the ozone layer has resulted at the poles, especially in the southern hemisphere
Recent models suggest the hole might not get larger
Why is there Seasonal Thinning of Ozone Over the Poles?
In 1984, researchers discovered 40-50% of the ozone in the upper stratosphere over Antarctica was being destroyed during the antarctic spring and early summer (Sept.-Dec.)
In 2000, ozone thinning above Antarctica was the largest ever and covered an area three times the size of the continental U.S. (11 million square miles)
Measurements indicate that CFCs are the primary culprits.
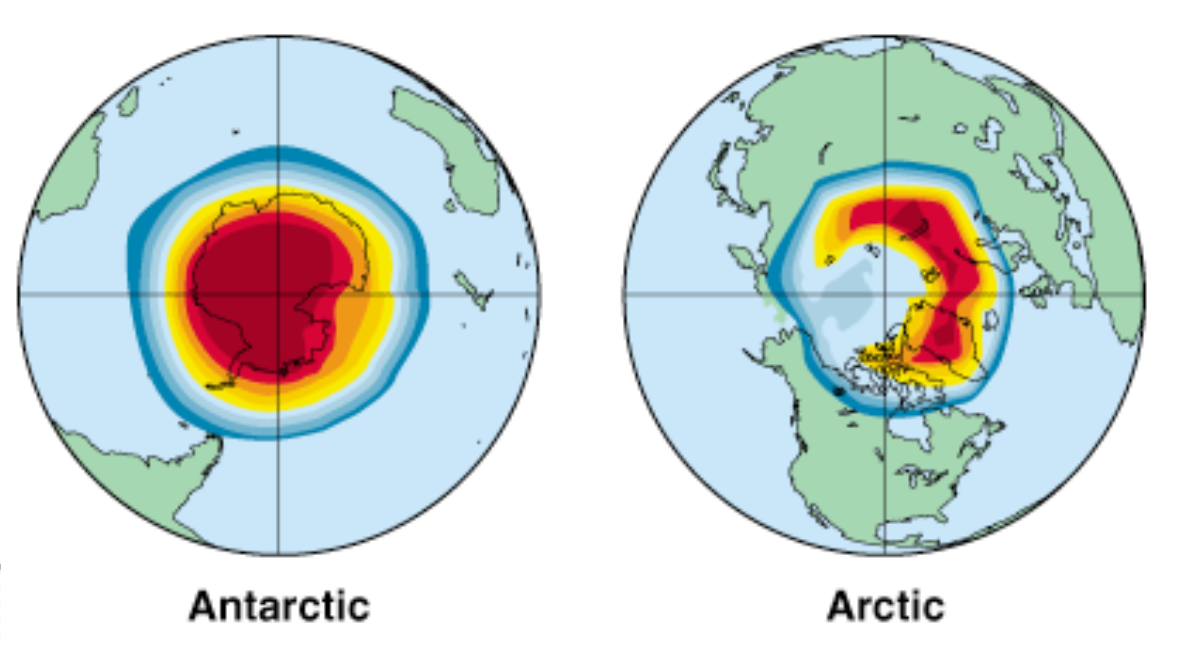
Ozone Loss
Projected total ozone loss, averaged over 2010-2019, during September for the Antarctic (left) and during March for the Arctic (right). Dark red represents ozone depletion of 54% or more; light blue, 18-30%; dark blue, 6-12%.
Solutions to Ozone Depletion
General
Phase out use of ozone–depleting chemicals (halons, CFCs, methyl chloroform, methyl bromide)
Phase in use of CFC substitutes [non–halogen aerosol propellants, hydrochlorofluorocarbons (HCFCs), hydrofluorocarbons (HFCs), hydrocarbons (HCs), ammonia, water & steam, terpenes, helium]
International Agreements
Montreal Protocol (1987)
Cut emission of CFCs by 35% by 2000
London (1990) and Copenhagen (1992)
Accelerate phase-out of other key ozone-depleting chemicals
World Meteorological Organization
Continued depletion for several decades
11-20 year time lag between when CFCs are released into the atmosphere and when they actually reach the stratosphere.
Persistence for decades
Return to 1980 levels by about 2050 and to 1950 levels by about 2100.
International agreements are followed
No major volcanic eruptions
Restoring the ozone layer may lead to an increase in global warming
Ozone depletion has been cooling the troposphere
Disguise as much as 30% of global warming caused by our greenhouse gas emissions.
How Can We Protect the Ozone Layer?
Technofixes
Huge radio-controlled blimps to form an electrical curtain.
Lasers blasting CFCs out of the atmosphere before they reach the stratosphere.
Montreal Protocol
Phase-out CFC emissions
Copenhagen Protocol
Phase-out CFC emissions and other ozone deleters
AP Environmental Science 9.1-9.2 - Ozone Depletion
What is the Ozone Shield?
Ozone Shield: a natural process that filters ultraviolet (UV) radiation before it reaches the lower atmosphere.
The layer of ozone gas (O3) in the upper stratosphere that screens out harmful ultraviolet radiation from the sun.
If the full amount of ultraviolet radiation falling on the stratosphere reached Earth’s surface; it is doubtful that any life could survive.
Ozone Depletion
How does Ozone Depletion Occur?
Overview
CFCs (clorofluorocarbons) emitted into atmosphere; they’re stable, move from troposphere to stratosphere
UV light breaks off chlorine molecule (Cl) from the CFC particle
Cl acts as a catalyst to break down ozone (O3)
catalyst – promotes a chemical reaction without itself being used up in the reaction
Shifts equilibrium of oxygen / ozone reaction
Summary of Reactions
Cl₃CF (example CFC) + UV → Cl+CCl₂
Cl+O₃ → ClO + O₂
ClO + O → Cl + O₂
This cycle repeats itself several times
“From Dream Chemicals to Nightmare Chemicals”
Thomas Midgley, Jr. A General Motors chemist, discovered the first chlorofluorocarbon (CFC) in 1930.
Family of highly useful CFCs – trichlorofluoromethane and dichlorodifluoromethane (AKA; freons)
Stable, odorless, nonflammable, nontoxic, and noncorrosive
Used in air conditioners, refrigerators, aerosol spray cans, cleaners for electronic parts, sterilants for hospital instruments, fumigants for granaries, bubbles in plastic foam used for packaging.
Consequences of Ozone Depletion
Humans
Increase in skin cancer & cataracts, especially in the southern hemisphere
More ozone near earth’s surface, produced in photochemical smog – lung problems, suppressed immune response, cancer
Threat of Ozone Depletion
Radiation from the sun includes ultraviolet (UV) radiation; UVA and UVB
UV radiation penetrates the atmosphere and is absorbed by biological tissues damaging protein and DNA molecules at the surfaces of all living things (sunburn).
Most of the dangerous UVB radiation (over 99%) is absorbed by ozone in the stratosphere.
Other Organisms
Primary Producers:
Reduction in phytoplankton
Lower crop yields
Decline in forest productivity
Animals:
Species disruption through increased exposure to UV-B radiation
Disruption of food chain
Effect of Ozone Depletion
Ozone Depleting Chemicals
Chlorofluorocarbons (CFCs)
Halons: fire extinguishers
Methyl bromide: fumigant
Carbon tetrachloride: cheap, highly toxic solvent
Methyl chloroform: cleaning solvent-clothes & metals
Hydrogen chloride; U.S. space shuttles
Ozone Hole
Seasonal thinning of the ozone layer has resulted at the poles, especially in the southern hemisphere
Recent models suggest the hole might not get larger
Why is there Seasonal Thinning of Ozone Over the Poles?
In 1984, researchers discovered 40-50% of the ozone in the upper stratosphere over Antarctica was being destroyed during the antarctic spring and early summer (Sept.-Dec.)
In 2000, ozone thinning above Antarctica was the largest ever and covered an area three times the size of the continental U.S. (11 million square miles)
Measurements indicate that CFCs are the primary culprits.
Ozone Loss
Projected total ozone loss, averaged over 2010-2019, during September for the Antarctic (left) and during March for the Arctic (right). Dark red represents ozone depletion of 54% or more; light blue, 18-30%; dark blue, 6-12%.

Solutions to Ozone Depletion
General
Phase out use of ozone–depleting chemicals (halons, CFCs, methyl chloroform, methyl bromide)
Phase in use of CFC substitutes [non–halogen aerosol propellants, hydrochlorofluorocarbons (HCFCs), hydrofluorocarbons (HFCs), hydrocarbons (HCs), ammonia, water & steam, terpenes, helium]
International Agreements
Montreal Protocol (1987)
Cut emission of CFCs by 35% by 2000
London (1990) and Copenhagen (1992)
Accelerate phase-out of other key ozone-depleting chemicals
World Meteorological Organization
Continued depletion for several decades
11-20 year time lag between when CFCs are released into the atmosphere and when they actually reach the stratosphere.
Persistence for decades
Return to 1980 levels by about 2050 and to 1950 levels by about 2100.
International agreements are followed
No major volcanic eruptions
Restoring the ozone layer may lead to an increase in global warming
Ozone depletion has been cooling the troposphere
Disguise as much as 30% of global warming caused by our greenhouse gas emissions.
How Can We Protect the Ozone Layer?
Technofixes
Huge radio-controlled blimps to form an electrical curtain.
Lasers blasting CFCs out of the atmosphere before they reach the stratosphere.
Montreal Protocol
Phase-out CFC emissions
Copenhagen Protocol
Phase-out CFC emissions and other ozone deleters
 Knowt
Knowt
