Unit 3 - Elements and the Periodic Table
Elements, Compounds, and Mixtures
Changes
Physical Changes - Don’t produce a new substance
Chemical Changes - Produces a new substance
Matter Classification
Pure Substances
Can’t be broken into simpler compounds without going through a chemical change
Made of atoms that are chemically bonded to each other
Elements - Pure substances made of only 1 type of atom
Compounds - Pure substances made of 2+ types of atoms
Have fixed ratios between components
Mixtures
Mixing 2+ substances that are NOT chemically combined
Can be separated through physical means
Distillation - Separating components in a mixture through the use of their differing boiling points
Chromatography - Separating components using differences in their ability to pass through substrates
Don’t have fixed ratios between components
Types of Mixtures
Homogeneous Mixture - The components combined are indistinguishable
Heterogeneous Mixture - The components combined are distinguishable
Atomic Numbers and Electron Configurations
Quantum Orbitals
Orbitals - Location in an atom where an electron could be
An atom can have any number of orbitals depending on the number of electrons they have
Each orbital can hold 2 electrons
Quantum Number - Describes the location of an electron / describes the orbital
Three main quantum numbers used to describe orbitals: “N“, “L“, and “M“
N - Principal quantum number; describes the size of the orbital
Must be >0
You can think of this as what ring in the Bohr model the orbital coincides with
L - Angular momentum quantum number; describes the orbital shape
Can be spherical, dumbbell/peanut, clover, etc shaped
Can be between 0 → N-1
M - Magnetic quantum number; describes orientation of the orbital
Can be between -L → +L
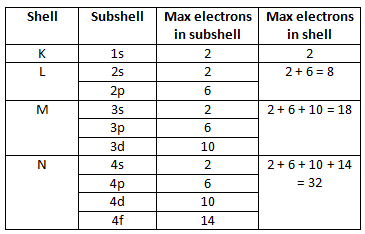
Shells & Subshells
Electron shell - A group of orbitals with the same principle quantum number (N)
Shells are filled consecutively from the center/lowest energy orbitals outward
Different shells can hold different numbers of electrons
Full shells are the most stable
Electron subshells - A group of orbitals with the same principle quantum number (N) AND angular momentum quantum number (L)
Subshell Classifications
L = 0 → S Orbital
L = 1 → P Orbital
L = 2 → D Orbital
L = 3 → F Orbital
The number of different values the magnetic quantum number (M) can be is equal to the number of subshells of a certain classification
The number of orbitals is equal to the number of different combinations of N, L, and M (Can be calculated with N^2)
To calculate the number of electrons a shell can hold, you just double this number, since each orbital can hold 2 electrons
This can also be calculated with the formula 2N^2
Electron Configuration - How electrons are positioned in an atom
Orbital Notation - A diagram that shows shells, subshells, and orbitals using lines & arrows
Lines represent orbitals
Numbers & letters at the bottom represent the name of the orbital
Arrows represent electrons
Upward and downward arrows represent a M subscript s value of either 1/2 or -1/2
Pauli Exclusion Principle - No 2 electrons can have identical quantum numbers
A fourth quantum number, M subscript s represents the quantum spin of a number
Can have a value of either -1/2 and 1/2
Only 2 values → only 2 electrons can be in a orbital, otherwise at least 1 pair of electrons will have identical quantum numbers
Hund’s Rule - Electrons are placed in individual orbitals before being paired
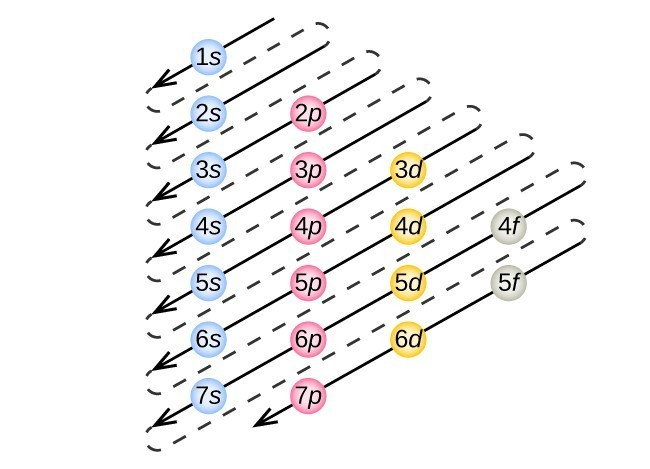
Aufbau Principle - Electrons fill orbitals from lowest energy → highest energy
This means electrons fill from lower N to higher N
D and F are the exception; 3d has higher energy than 4s, so 4s will fill before 3d.
Follow the diagonal rule to determine order in which orbitals are filled
The History and Arrangement of the Periodic Table
Antoine Lavosier
Wrote “Elementary Treatise of Chemistry“ in 1789
Considered the world’s first modern chemistry textbook
Classified elements into 4 groups:
Acid-making
Gas-like
Wrongly classified light & heat as elements
Metallic
Earthy
Almost entirely made up of compounds
John Dobereiner
Arranged elements w/ similar properties into triads (groups of 3)
Difference between mass of elements 1 & 2 is about equal to difference in mass between 2 & 3
John Newlands
Arranged elements by atomic mass
Established “law of octaves“
Repeating pattern of similar properties every 8 elements
Dimitri Mendeleev
Created the first iteration of the modern periodic table
Arranged elements by atomic mass
Organized table rows/columns by chemical properties
Henry Moseley
Arranged elements by atomic number
Account for variation in natural isotopes
Periodic Table
Organized by atomic number (number of protons)
Columns have similar chemical properties due to having the same number of valence (outer) electrons
Each row is a new shell
Periods - A row on the periodic table
Atomic number increases from left to right
Chemical properties systematically change
Groups/Families - A column on the periodic table
All elements in groups have similar chemical properties
Cells - Give information about an element
Atomic number
Atomic mass
Atomic symbol
Element name
Elements
Natural: Elements 1-94
Man-Made: Elements 95-118
Metals: Left of the “staircase“ except hydrogen
Malleable
Ductile
Conduct heat & electricity
Mostly solids
Semi-Metals/Metalloids: The “staircase”
Properties of both groups
Non-Metals: Right of the “staircase“ plus hydrogen
Brittle
Poor Conductors
Can be any state
Main Group Elements
Alkali Metals - Group 1
Silver colored
Soft
Highly reactive with water/oxygen
Oxidizes in air
Alkaline Earth Metals - Group 2
Silver colored
More brittle than alkaline metals
Somewhat reactive
Low density, melting, and boiling points
Halogens - Group 17
Highly reactive w/ metals
Form salts
Toxic to most organisms
Mostly occur as diatomic molecules
Noble Gases - Group 18
Stable; don’t bond w/ other atoms
Non-flammable
Extremely low boiling points
Used in lights, produces colors when excited
Transition Metals
Form colored compounds
Some have unique properties
Some are magnetic
Some are very reactive
Inner Transition Metals
Can be radioactive
Lanthanides
Actinides
Electrons and the Periodic Table
Noble Gas Notation
Using noble gases to represent filled shells in longhand electron configuration
Separates valence and non-valence(core) electrons in an atom
Valence Electrons
The number of electrons on the outer shell of an atom
Determines the chemical properties of the atom
Correlated with the groups that the element is in in the periodic table
Group number = number of valence electrons
Determining Valence Electrons
Periods 1-3
Group number / highest S and P orbitals
Periods 4+
Highest S and P orbitals + partially filled d and f orbitals
Periodic Table & Orbitals
S-Block Elements - Elements in groups 1 & 2 + Helium
Has valence electrons in the S orbital
P-Block Elements - Elements in groups 13 → 18 - Helium
Rows 1-3
Has valence electrons in the S and P orbitals, with the last added electron being in the P orbital
Rows 4+
Has valence electrons in the S, D, and P orbitals, with “N“ of the D subshell being 1 less than the N of the S & P subshells and the last added electron being in the P orbital
D-Block Elements - Elements in groups 3 → 12 + Lutetium and Lawrencium
Has valence electrons in the S and D orbitals, with “N“ of the D subshell being 1 less than the N of the S subshell and the last added electron being in the D orbital
F-Block Elements - Lanthanides & Actinides - Lutetium and Lawrencium
Has valence electrons in the S and F orbitals, with “N“ of the F subshell being 2 less than the N of the S subshell and the last added electron being in the F orbital
Exceptions
Chromium
Predicted - [Ar] 4s^2 3d^4
Actual - [Ar] 4s^1 3d^5
Often D & F block elements that are transition metals
Happens because electrons fill lowest energy shell
Periodic Trends
Atomic Radius - 1/2 the distance between two identical atoms in a diatomic molecule
Increases down a group
Decreases across a row
More protons → electrons are pulled slightly closer together
Ionic Radius - Measure of the size of an ion
Anion - Negative ions (atoms that gain electrons)
Larger; More electrons cause more electron repulsion
Cation - Positive ions (atoms that lose electrons)
Smaller; less electrons cause less electron repulsion
Increase down a group
Decrease for cations across a row
Decrease for anions across a row
Increase when switching from cations to anions across a period
Ionization Energy - The energy required to remove an electron from an atom in a gas phase
Changes based
Nuclear charge
Distance from nucleus
The number of already removed electrons
First Ionization Energy - Energy needed to remove 1 electron from an atom
Second ionization energy - amount of energy to remove another electron after the first one is removed, etc
Main Group Elements
Increases across periods
Decreases down groups
Decreases between groups 2 & 13 and groups 15 & 16
Electron Affinity - Energy required to add an electron to a neutral atom in a gas phase
Decreases across a period
Increases down a group
Electronegativity - How much an atom attracts other electrons from other atoms
Increase across a period
Decrease down a group
Unit 3 - Elements and the Periodic Table
Elements, Compounds, and Mixtures
Changes
Physical Changes - Don’t produce a new substance
Chemical Changes - Produces a new substance
Matter Classification
Pure Substances
Can’t be broken into simpler compounds without going through a chemical change
Made of atoms that are chemically bonded to each other
Elements - Pure substances made of only 1 type of atom
Compounds - Pure substances made of 2+ types of atoms
Have fixed ratios between components
Mixtures
Mixing 2+ substances that are NOT chemically combined
Can be separated through physical means
Distillation - Separating components in a mixture through the use of their differing boiling points
Chromatography - Separating components using differences in their ability to pass through substrates
Don’t have fixed ratios between components
Types of Mixtures
Homogeneous Mixture - The components combined are indistinguishable
Heterogeneous Mixture - The components combined are distinguishable
Atomic Numbers and Electron Configurations
Quantum Orbitals
Orbitals - Location in an atom where an electron could be
An atom can have any number of orbitals depending on the number of electrons they have
Each orbital can hold 2 electrons
Quantum Number - Describes the location of an electron / describes the orbital
Three main quantum numbers used to describe orbitals: “N“, “L“, and “M“
N - Principal quantum number; describes the size of the orbital
Must be >0
You can think of this as what ring in the Bohr model the orbital coincides with
L - Angular momentum quantum number; describes the orbital shape
Can be spherical, dumbbell/peanut, clover, etc shaped
Can be between 0 → N-1
M - Magnetic quantum number; describes orientation of the orbital
Can be between -L → +L
Shells & Subshells
Electron shell - A group of orbitals with the same principle quantum number (N)
Shells are filled consecutively from the center/lowest energy orbitals outward
Different shells can hold different numbers of electrons
Full shells are the most stable
Electron subshells - A group of orbitals with the same principle quantum number (N) AND angular momentum quantum number (L)
Subshell Classifications
L = 0 → S Orbital
L = 1 → P Orbital
L = 2 → D Orbital
L = 3 → F Orbital

The number of different values the magnetic quantum number (M) can be is equal to the number of subshells of a certain classification
The number of orbitals is equal to the number of different combinations of N, L, and M (Can be calculated with N^2)
To calculate the number of electrons a shell can hold, you just double this number, since each orbital can hold 2 electrons
This can also be calculated with the formula 2N^2
Electron Configuration - How electrons are positioned in an atom
Orbital Notation - A diagram that shows shells, subshells, and orbitals using lines & arrows
Lines represent orbitals
Numbers & letters at the bottom represent the name of the orbital
Arrows represent electrons
Upward and downward arrows represent a M subscript s value of either 1/2 or -1/2
Pauli Exclusion Principle - No 2 electrons can have identical quantum numbers
A fourth quantum number, M subscript s represents the quantum spin of a number
Can have a value of either -1/2 and 1/2
Only 2 values → only 2 electrons can be in a orbital, otherwise at least 1 pair of electrons will have identical quantum numbers
Hund’s Rule - Electrons are placed in individual orbitals before being paired
Aufbau Principle - Electrons fill orbitals from lowest energy → highest energy
This means electrons fill from lower N to higher N
D and F are the exception; 3d has higher energy than 4s, so 4s will fill before 3d.
Follow the diagonal rule to determine order in which orbitals are filled
The History and Arrangement of the Periodic Table
Antoine Lavosier
Wrote “Elementary Treatise of Chemistry“ in 1789
Considered the world’s first modern chemistry textbook
Classified elements into 4 groups:
Acid-making
Gas-like
Wrongly classified light & heat as elements
Metallic
Earthy
Almost entirely made up of compounds
John Dobereiner
Arranged elements w/ similar properties into triads (groups of 3)
Difference between mass of elements 1 & 2 is about equal to difference in mass between 2 & 3
John Newlands
Arranged elements by atomic mass
Established “law of octaves“
Repeating pattern of similar properties every 8 elements
Dimitri Mendeleev
Created the first iteration of the modern periodic table
Arranged elements by atomic mass
Organized table rows/columns by chemical properties
Henry Moseley
Arranged elements by atomic number
Account for variation in natural isotopes
Periodic Table
Organized by atomic number (number of protons)
Columns have similar chemical properties due to having the same number of valence (outer) electrons
Each row is a new shell
Periods - A row on the periodic table
Atomic number increases from left to right
Chemical properties systematically change
Groups/Families - A column on the periodic table
All elements in groups have similar chemical properties
Cells - Give information about an element
Atomic number
Atomic mass
Atomic symbol
Element name
Elements
Natural: Elements 1-94
Man-Made: Elements 95-118
Metals: Left of the “staircase“ except hydrogen
Malleable
Ductile
Conduct heat & electricity
Mostly solids
Semi-Metals/Metalloids: The “staircase”
Properties of both groups
Non-Metals: Right of the “staircase“ plus hydrogen
Brittle
Poor Conductors
Can be any state
Main Group Elements
Alkali Metals - Group 1
Silver colored
Soft
Highly reactive with water/oxygen
Oxidizes in air
Alkaline Earth Metals - Group 2
Silver colored
More brittle than alkaline metals
Somewhat reactive
Low density, melting, and boiling points
Halogens - Group 17
Highly reactive w/ metals
Form salts
Toxic to most organisms
Mostly occur as diatomic molecules
Noble Gases - Group 18
Stable; don’t bond w/ other atoms
Non-flammable
Extremely low boiling points
Used in lights, produces colors when excited
Transition Metals
Form colored compounds
Some have unique properties
Some are magnetic
Some are very reactive
Inner Transition Metals
Can be radioactive
Lanthanides
Actinides
Electrons and the Periodic Table
Noble Gas Notation
Using noble gases to represent filled shells in longhand electron configuration
Separates valence and non-valence(core) electrons in an atom
Valence Electrons
The number of electrons on the outer shell of an atom
Determines the chemical properties of the atom
Correlated with the groups that the element is in in the periodic table
Group number = number of valence electrons
Determining Valence Electrons
Periods 1-3
Group number / highest S and P orbitals
Periods 4+
Highest S and P orbitals + partially filled d and f orbitals
Periodic Table & Orbitals
S-Block Elements - Elements in groups 1 & 2 + Helium
Has valence electrons in the S orbital
P-Block Elements - Elements in groups 13 → 18 - Helium
Rows 1-3
Has valence electrons in the S and P orbitals, with the last added electron being in the P orbital
Rows 4+
Has valence electrons in the S, D, and P orbitals, with “N“ of the D subshell being 1 less than the N of the S & P subshells and the last added electron being in the P orbital
D-Block Elements - Elements in groups 3 → 12 + Lutetium and Lawrencium
Has valence electrons in the S and D orbitals, with “N“ of the D subshell being 1 less than the N of the S subshell and the last added electron being in the D orbital
F-Block Elements - Lanthanides & Actinides - Lutetium and Lawrencium
Has valence electrons in the S and F orbitals, with “N“ of the F subshell being 2 less than the N of the S subshell and the last added electron being in the F orbital
Exceptions
Chromium
Predicted - [Ar] 4s^2 3d^4
Actual - [Ar] 4s^1 3d^5
Often D & F block elements that are transition metals
Happens because electrons fill lowest energy shell
Periodic Trends
Atomic Radius - 1/2 the distance between two identical atoms in a diatomic molecule
Increases down a group
Decreases across a row
More protons → electrons are pulled slightly closer together
Ionic Radius - Measure of the size of an ion
Anion - Negative ions (atoms that gain electrons)
Larger; More electrons cause more electron repulsion
Cation - Positive ions (atoms that lose electrons)
Smaller; less electrons cause less electron repulsion
Increase down a group
Decrease for cations across a row
Decrease for anions across a row
Increase when switching from cations to anions across a period
Ionization Energy - The energy required to remove an electron from an atom in a gas phase
Changes based
Nuclear charge
Distance from nucleus
The number of already removed electrons
First Ionization Energy - Energy needed to remove 1 electron from an atom
Second ionization energy - amount of energy to remove another electron after the first one is removed, etc
Main Group Elements
Increases across periods
Decreases down groups
Decreases between groups 2 & 13 and groups 15 & 16
Electron Affinity - Energy required to add an electron to a neutral atom in a gas phase
Decreases across a period
Increases down a group
Electronegativity - How much an atom attracts other electrons from other atoms
Increase across a period
Decrease down a group
 Knowt
Knowt