Cell Bio- Exam 2
Slide Set 5: Vesicular Traffic, Secretion, and Endocytosis
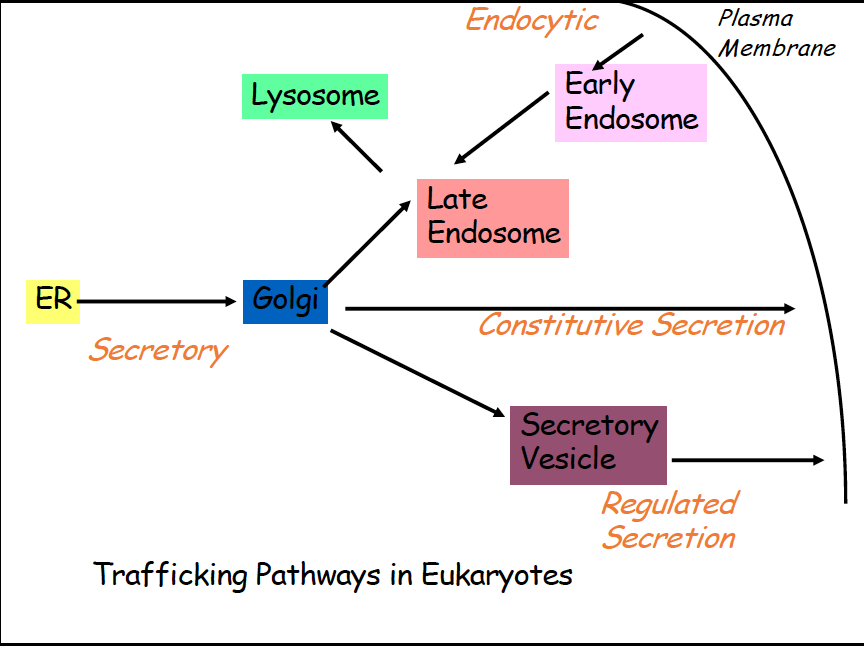
Vesicular Transport
Proteins are synthesized in the ER, then are moved from ER to golgi, once mature proteins are formed, they need to leave the ER (Secretory)
After golgi, they have multiple different pathways
Constitutive secretion- constant secretion of proteins from cell, golgi to out of cell
regulated secretion- secretory vesicle takes protein out of cell from golgi
Endocytic- early endosome takes proteins from membrane to late endosome and then sometimes to lysosome
Microscopy study with GFP
studied trafficking via GFP virus particles
use temperature, if temp inc, protein mvmt blocked
you can track proteins via fluorescent microscopy
results: there is trafficking within the cell, you can get a rough est of the time that it takes
tracking total fluorescence signal over time
Oligosaccaride modification
mannose trimming occurs when oligosaccaride moves from ER to golgi
treated with endoglycosidase D which cleaves sugar from protein
Vesicle Budding and Fusion
transport vesicle leaves donor compartment
transport vesicle fuses with target compartment
Coated Vesicle Budding
SNARE protein helps transport vesicles recognize target membranes
membrane cargo protein and soluble cargo protein bind together
coat proteins surround vesicle
Uncoated vesicle fusion
V SNARE proteins will interact with T SNARE proteins on membrane
Rabs protein- can help recognize which target mem they should fuse too, assists with docking
What is the mechanism by which vesicles are formed?
Three types of coated vesicles
Clathrin coated - helps with transport from trans golgi network to late endosome and helps transports obj entering the cell via endocytosis
have heavy and light chains, as well as binding site for assembly particles
soccer ball structure
Functions:
help form mechanical force to form vesicle
coat subunits bind to surface of donor membrane
clathrin and other proteins help form bud/vesicle and help with the mechanical force of budding off
capture membrane receptors
clathrin and adaptin (bound together) bind to cargo receptor bound to cargo molecules in membrane, and then start budding process,
adaptin helps transmem receptor bind to coating proteins
certain aa are carried that signals adaptin to bind, these are then phosphorylated
Dynamin
required for pinching off of clathrin vesicles from donor membrane
polymerizes around the neck and then hydrolyzes GTP, conformational change initiated in dynamin that stretches vesicle neck until the vesicle pinches off
COP 1- in charge of moving protein from trans golgi back to ER
coatomer coated
intra golgi traffic, golgi to ER
ARF plays a role in coat formation
COP 2- helps with protein leaving ER to cis golgi
coatomer coated
Sar 1 uses COP 2 components
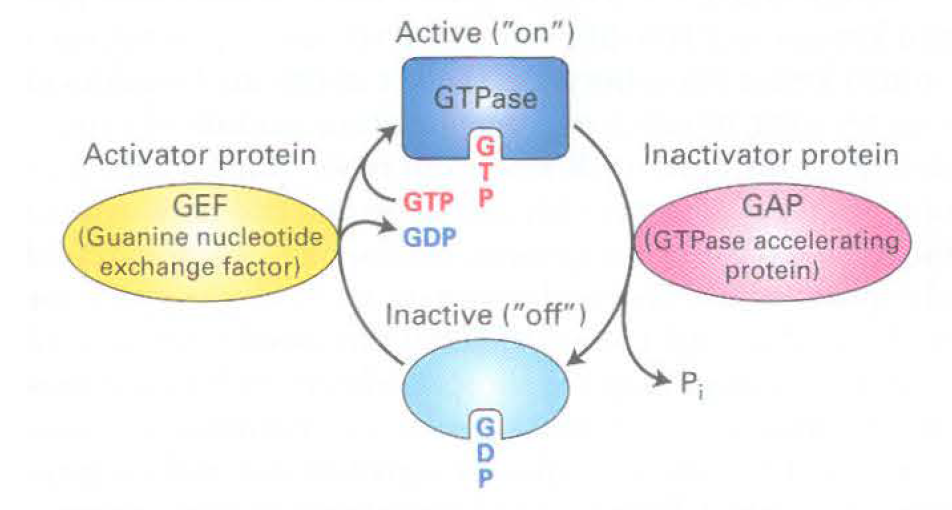
GTPases
Active- when protein binds to GTP
GAP- hydrolyzes GTP to GDP
Sar 1 initially binds to GTP, then binds to Sec 12 to hydrolyze GTP, then recruits COP2 components to have GTP bound to mem
Sar 1- controls coat assembly on COP2 vesicles
inactive- off, GDP bound
GEF- releases GDP so GTP can be made
ARF- also a GTPase, plays role in coat formation in COP1 and Clathrin coated vesicles, intitially binds to GDP
What are the molecular signals on vesicles that cause them to bind only to the appropriate target membrane?
SNARES and RAB GTPases play a role in vesicle traffic and fusion
generate tight interactions, help vesicles fuse to the donor membrane
RAB GTPase
donor mem: RAB receptor, vesicle: RAB
mediate diff transport vesicles fused to diff transport membranes
many diff RABs in eukaryotic cells
How do transport vesicles and their target organelles fuse?
SNARE and RAB help vesicle recognize donor membrane
RAB will not help fuse, will help recognize membrane
Vesicle Fusion Machinery
Vesicle Docking: V SNARE and T SNARE associate, RAB binds to RAB receptor
Assembly of SNARE complex:
SNAP 25- snare complex, includes V SNARE and Syntaxin
generates strong force to help fusion to the membrane
twisted very tightly together
Membrane Fusion
proteins work to untwist SNAP 25
fusion of membranes occurs
Disassembly of SNARE complexes
SNARE complexes disassociate and are free for another round of vesicle fusion, RAB also disassociates from the RAB effector
Steps in Secretory Pathway cont
Vesicular Transport from ER to Golgi
protein always goes from cis to trans face of golgi
cis cisterna→ medial cisterna → trans cisterna
ER retention signal- four aa, KDEL; if added at c term of protein it will return to ER from cis golgi bc it will bind to place on cis golgi and be recognized
Cisternal progression through golgi glycosylation and other mods in golgi
removal of 3 mannose residues in cis golgi (-3 Man)
protein moves to medial golgi by cisternal maturation
3 GlcNAc residues added , 2 more mannose removed, single fucose is added (+ 3 GlcNAc, -2Man, + Fucose)
processing completed in trans golgi by addition of 3 galactose residues and linkage of N-acetylneuraminic acid residue to each galactose (+3 Gal, + 3 NANA)

Role of glycosylation
post translational modification
helps protein become hydrophilic→ aids in folding
aid in transport (rarely- targeting to lysosome)
resistance to proteases (stability)
protein protein interactions
Vesicular sorting at trans- golgi network
Vesicular Trafficking to Final Destination (golgi to ___)
Endosome
Plasma Mem
constitutive secretion- unregulated membrane fusion
regulated secretion- regulated membrane fusion
Lysosome
some proteins go here
very acidic environment
v class pumps used with ATP to pump proton inside
lysosomes form a functional hub for cellular trrafficking pathways
ER→ Golgi→ lysosome
Pinocytosis→ lysosome
Phagocytosis→ lysosome
autophagy→ lysosome
How does cell know which proteins are sent to the lysosome?
M6P residues!
receptor on trans golgi network that will bind to M6P and will incorporate into vesicle and then will go to late endosome
if pH low in late endosome, M6P transferred to lysosome
Lysosomal Storage diseases
can be due to absence of 1 or more lysosomal hydrolases or the mistargeting of lysosomal hydrolases
characterized by tissue destruction or accumulation of undigested macromolecules
I cell- protein stuck in trans golgi, severe tissue destruction, GlcNac Deficiency
Endocytosis
goes through plasma mem, through early endosome then late endosome, then lysosome
pinocytosis-
very tiny things; proteins, lipids. Goes through early, late, then lysosome
continuous process, rate depends on cell type
pinocytotic vesicle forms from clathrin coated pits in plasma mem
receptor mediated endocytosis- ligand binds to cell surface receptor, clathrin helps to form vesicle, clathrin coats vesicle
phagocytosis-
large things like bacteria; phagosome then to lysosome
feeding for lower single celled euks
multi celled orgs- used as a defense against invading microbes
requires surface receptors, triggered event
autophagy-
from ER, if we do not need certain organelles anymore, autophagosome forms then transported to lysosome
LDL Uptake
LDL- byproduct of fat transport, have ApoB protein
ApoB and LDL receptor bind
vesicle begins to form with help of clathrin coat
transported to early endosome→ late endosome→ lysosome
Disorders- LDL receptor missing, receptors do not associate with clathrin coat
Fate of cell surface receptors after endocytosis
recycling of receptor to same domain
receptor transported back to surface of membrane and pH will change→ receptor ready to bind to another LDL particle
degradation of receptor after endocytosis
in lysosome
transcytosis
any protein that is missent to basolateral side will be resent to apical membrane side
the vesicular transport of macromolecules from one side of a cell to the other
Slide Set 6: Microfilaments
The Cytoskeleton
Functions of cytoskeleton
cell shape, mvmt, and contraction
organelle mvmt and organization
cell division
intracellular org and vesicle mvmt
interacting with signaling pathways
basically like the bones of the cell
Components
Microfilaments
actin filaments, thinner
Microtubules
tubulin dimers, thicker
Intermediate filaments
various, diff proteins combined together
Cell signaling
signals tell cytoskeleton abt organization and mvmt of organelles as well as changes in cell shape, mvmt, and contraction
Actin Microfilaments
Functions
org of intracellular organelles and transport of vesicles (myosin)
intracellular mobility (bacteria)
cellular stability
cellular motility
muscle contraction
Lamellipodium
supported by growth of actin filaments, generates a protrusion structure to adhere to surface and move cell forward
Polymerization and Dynamics
1 actin filament= 2 strands
one + end (0.12 M), one - end (0.6)
g actin is monomer, microfilament polymer of actin
ATP binding cleft in actin structure
alpha, gamma, and beta actin: all associated with diff structures
G actin polymerization
g actin binds to f actin, elongating existing filament
can be added to + and - end, and leave from both sides
g actin dec to critical con→ polymer shrink
g actin inc above critical conc→ polymer inc in length
Actin Binding Proteins
Polymerization- Profilin and Thymosin B4
Profilin- promotes polymerization
Thymosin b4- blocks polymerization of ATP
Length- Cofilin, Gelsolin
Nucleation and branching- Arp2/3
Crosslinking- Filamin
Motor Proteins- myosin
stability/cap end of filaments- capz and tropomodulin
CapZ- caps at + end
Tropomodulin- caps at - end
org of filaments/muscle contraction, binds to side of filaments- nebulin
Actin based Motility
Formin - leads to assembly for long actin filaments
will form dimer structure
actin binds to structure and elongation commences
ARP2/3 complex can be used for polymerization to power motility, mediates branching
listeria monocytogenes uses actin polymerization to move through cells and from cell to cell→ hijack actin machinery and polymerize it to move around
ActA protein activates Arp2/3 to nucleate new filament assembly from preexisting filaments
filaments grow at + end until capped by Cap Z
actin recycled through cofilin, which enhances depolymerization at the - end of the filaments
this process propels bacterium forward
Toxins that perturb pool of actin monomers
Cytochalasin D- depolymerizes actin by blocking further addition of subunits
Latrunculin- inhibits g actin from adding to filament end
Jasplakinolide- stabilizes and binds actin dimers, lowers critical conc bar
Phalloidin- prevents actin filaments from depolymerizing by locking F subunits together
Actin also interacts with itself
types of lateral attachment of microfilaments to membranes
ankyrin- binds to Band 3 and then spectrin, forms network
band 4.1
Actin Motor Proteins
myosin
can bind to actin and help generate contraction in muscle cells
composed of heavy chains and light chains, diff myosin has diff amts of each
myosin heads can bind to ATP and actin
Myosin 1
small, single head
step size 10-14nm
works with membrane association and endocytosis
Myosin 2
dimer (2 heavy chain)
8nm step
bipolar filaments
works with contractions
Myosin 5
bigger, 2 heavy chains and more light chains
36nm step
responsible for organelle transport
Myosin mvmt process
ATP binds to head grp, head group not associated with actin yet
ATP hydrolyzed, head grp rotated into position to bind, head grp binds to actin
power stroke occurs, Pi released and myosin straightened, moving actin filament left
ADP released, the ATP bound and head grp released from actin
Step size vs neck length
is myosin step size/velocity proportional to neck length?
YES, velocity inc with inc neck length
contractile ring
myosin 2 takes a large part in forming when cells are splitting, myosin 1 is on outside of cells
Sarcomere (not protein, just structure of skeletal muscle)
vertical component is Z band, in between is A band, myosin in between actin filaments
actin end facing inside is - end
sarcoplasmic reticulum- specialized region of the ER, regulates and stores Ca (Ca helps muscle cells to contract)
Cap Z- binds to + end of actin
Tropomodulin- binds to - end of actin
Nebulin- binds to side of actin filaments
Titin- binds to myosin and Z disk proteins
Rho GTPases
membrane bound Rho proteins can bind effector proteins that cause changes in the actin cytoskeleton
dominant active rho- always keep making actin
Cdc42- filopodia formation
works at the front of cell, activates Rac
guys see a Rac and are activated
RacGTP- lamellipodia formation
leads to activation of Arp2/3 and Rho
RhoGTP- Stress fiber formation
leads to myosin 2 activation
Slide Set 7: Microtubules and Intermediate filaments
MIcrofilaments vs Microtubules vs Intermediate filaments
microfilaments
actin binds ATP
form rigid gels, networks, and bundles
tracks for myosin
contractile machinery and network at cell cortex
microtubules
tubulin binds GTP, rigid and not easily bent
trasks for kinesins and dyesins
organization for long range organelles
Intermediate filaments
great tensile strength, less dynamic, unpolarized
no motors
cell and tissue integrity
Microtubules
play a role in….
organization of organelles and transport of vesicles
mvmt of cilia and flagella
nerve cell, RBC, and flagellar structure
alignment and separation of chrom during mitosis
Tubulin
monomer of microtubules, alpha and beta make up monomer
Two populations of microtubules
Unstable short lived- assembles and disassembles rapidly
stable and long lived- remain polymerized for a long time (sperm flagella, RBC, nerve cells)
Polymerization and Structure of Microtubules
Structure
tubulin has alpha and beta parts
bind to 2 GTP
alpha T GTP is never hydrolyzed
beta T GTP can be hydrolyzed
one end is beta T exposed→ + end
one end is alpha T exposed → - end
microtubules made up of 13 protofilaments → singlet
can have doublets (cilia/flagella) and triplets (basal bodies and centrioles) as well
Polymerization
microtubules assembled from MTOC
MTOC-any structure used by cells to nucleate and organized microtubules
centrosome falls into this category
neg end of microtubules at MTOC
gamma tubulin ring nucleates microtubule assembly
Dynamics of Microtubules
Length over time: Assembly stage→ Catastrophe stage→ Disassembly stage→ Rescue Stage
Polymerization of tubulin into microtubules
protofilament first formed
alpha T first binds to protofilament, then beta T
sheet assembly
then form tube formation
GTP cap at top, GDP microtubule is the rest
GTP cap bc alpha and beta T carry GTP, addition of another Alpha and beta T will cause hydrolysis
end more smooth (assembly), - end more rough (disassembly)
Disassembly and reassembly of microtubules
cool to 4 deg, microtubule will disassemble
warm to 37 deg the microtubule will repolarize
Drugs that disrupt microtubule dynamics
colchincine- binds btwn alpha and beta T dimer so it cannot be used for polymerization, causes depolymerization
taxol- bind to side of tubules - stabilize the microtubule structure
Binding Proteins
MAPs
can stabilize microtubules, similar to taxol
side binding
MAP2- longer
Tau- shorter
+TIPS
can regulate + end of microtubules
Motors
Kinesins
ferry cargo around the cell
ferry towards + end
have light chain, bind to ATP for energy resource
bind to microtubule with head groups, bind to vesicle via kinesin receptor
hydrolyze ATP to drive mvmt
Kinesin 1 and 2- organelle, mRNA, and chromosome transport
Kinesin 5- bipolar structure, 2 head grps, can bind to 2 diff microtubules, microtubule sliding
Kinesin 13- can regulate microtubule end disassembly
Process
first head group, (leading head), no ATP, bound to microtubule
leading head then binds to ATP
conformational change induced, following head swings forwards
following head becomes leading head
new leading head releases ADP which it was originally bound to, and new following head hydrolyzes ATP to ADP and then process restarts
Dyneins
ferry cargo around the cell towards - end
Power stroke of dynein- ATP hydrolysis causes change in orientation of head→ mvmt of MT
dynactin- bind cargo, make dynein more processive
LIS1 protein- interact with ATPase domain of dynein to elongate power stroke
Intermediate filaments
heterogeneous
great tensile strength
no known motors use them as tracks
more stable than filaments or tubules
no intrinsic polarity
made up of protofilaments that can form diff structures
have N term and c term and head and tail end
keratin, lamin, vimentin
Cell Bio- Exam 2
Slide Set 5: Vesicular Traffic, Secretion, and Endocytosis
Vesicular Transport
Proteins are synthesized in the ER, then are moved from ER to golgi, once mature proteins are formed, they need to leave the ER (Secretory)
After golgi, they have multiple different pathways
Constitutive secretion- constant secretion of proteins from cell, golgi to out of cell
regulated secretion- secretory vesicle takes protein out of cell from golgi
Endocytic- early endosome takes proteins from membrane to late endosome and then sometimes to lysosome

Microscopy study with GFP
studied trafficking via GFP virus particles
use temperature, if temp inc, protein mvmt blocked
you can track proteins via fluorescent microscopy
results: there is trafficking within the cell, you can get a rough est of the time that it takes
tracking total fluorescence signal over time
Oligosaccaride modification
mannose trimming occurs when oligosaccaride moves from ER to golgi
treated with endoglycosidase D which cleaves sugar from protein
Vesicle Budding and Fusion
transport vesicle leaves donor compartment
transport vesicle fuses with target compartment
Coated Vesicle Budding
SNARE protein helps transport vesicles recognize target membranes
membrane cargo protein and soluble cargo protein bind together
coat proteins surround vesicle
Uncoated vesicle fusion
V SNARE proteins will interact with T SNARE proteins on membrane
Rabs protein- can help recognize which target mem they should fuse too, assists with docking
What is the mechanism by which vesicles are formed?
Three types of coated vesicles
Clathrin coated - helps with transport from trans golgi network to late endosome and helps transports obj entering the cell via endocytosis
have heavy and light chains, as well as binding site for assembly particles
soccer ball structure
Functions:
help form mechanical force to form vesicle
coat subunits bind to surface of donor membrane
clathrin and other proteins help form bud/vesicle and help with the mechanical force of budding off
capture membrane receptors
clathrin and adaptin (bound together) bind to cargo receptor bound to cargo molecules in membrane, and then start budding process,
adaptin helps transmem receptor bind to coating proteins
certain aa are carried that signals adaptin to bind, these are then phosphorylated
Dynamin
required for pinching off of clathrin vesicles from donor membrane
polymerizes around the neck and then hydrolyzes GTP, conformational change initiated in dynamin that stretches vesicle neck until the vesicle pinches off
COP 1- in charge of moving protein from trans golgi back to ER
coatomer coated
intra golgi traffic, golgi to ER
ARF plays a role in coat formation
COP 2- helps with protein leaving ER to cis golgi
coatomer coated
Sar 1 uses COP 2 components
GTPases
Active- when protein binds to GTP
GAP- hydrolyzes GTP to GDP
Sar 1 initially binds to GTP, then binds to Sec 12 to hydrolyze GTP, then recruits COP2 components to have GTP bound to mem
Sar 1- controls coat assembly on COP2 vesicles
inactive- off, GDP bound
GEF- releases GDP so GTP can be made
ARF- also a GTPase, plays role in coat formation in COP1 and Clathrin coated vesicles, intitially binds to GDP
What are the molecular signals on vesicles that cause them to bind only to the appropriate target membrane?
SNARES and RAB GTPases play a role in vesicle traffic and fusion
generate tight interactions, help vesicles fuse to the donor membrane
RAB GTPase
donor mem: RAB receptor, vesicle: RAB
mediate diff transport vesicles fused to diff transport membranes
many diff RABs in eukaryotic cells
How do transport vesicles and their target organelles fuse?
SNARE and RAB help vesicle recognize donor membrane
RAB will not help fuse, will help recognize membrane
Vesicle Fusion Machinery
Vesicle Docking: V SNARE and T SNARE associate, RAB binds to RAB receptor
Assembly of SNARE complex:
SNAP 25- snare complex, includes V SNARE and Syntaxin
generates strong force to help fusion to the membrane
twisted very tightly together
Membrane Fusion
proteins work to untwist SNAP 25
fusion of membranes occurs
Disassembly of SNARE complexes
SNARE complexes disassociate and are free for another round of vesicle fusion, RAB also disassociates from the RAB effector
Steps in Secretory Pathway cont
Vesicular Transport from ER to Golgi
protein always goes from cis to trans face of golgi
cis cisterna→ medial cisterna → trans cisterna
ER retention signal- four aa, KDEL; if added at c term of protein it will return to ER from cis golgi bc it will bind to place on cis golgi and be recognized
Cisternal progression through golgi glycosylation and other mods in golgi
removal of 3 mannose residues in cis golgi (-3 Man)
protein moves to medial golgi by cisternal maturation
3 GlcNAc residues added , 2 more mannose removed, single fucose is added (+ 3 GlcNAc, -2Man, + Fucose)
processing completed in trans golgi by addition of 3 galactose residues and linkage of N-acetylneuraminic acid residue to each galactose (+3 Gal, + 3 NANA)

Role of glycosylation
post translational modification
helps protein become hydrophilic→ aids in folding
aid in transport (rarely- targeting to lysosome)
resistance to proteases (stability)
protein protein interactions
Vesicular sorting at trans- golgi network
Vesicular Trafficking to Final Destination (golgi to ___)
Endosome
Plasma Mem
constitutive secretion- unregulated membrane fusion
regulated secretion- regulated membrane fusion
Lysosome
some proteins go here
very acidic environment
v class pumps used with ATP to pump proton inside
lysosomes form a functional hub for cellular trrafficking pathways
ER→ Golgi→ lysosome
Pinocytosis→ lysosome
Phagocytosis→ lysosome
autophagy→ lysosome
How does cell know which proteins are sent to the lysosome?
M6P residues!
receptor on trans golgi network that will bind to M6P and will incorporate into vesicle and then will go to late endosome
if pH low in late endosome, M6P transferred to lysosome
Lysosomal Storage diseases
can be due to absence of 1 or more lysosomal hydrolases or the mistargeting of lysosomal hydrolases
characterized by tissue destruction or accumulation of undigested macromolecules
I cell- protein stuck in trans golgi, severe tissue destruction, GlcNac Deficiency
Endocytosis
goes through plasma mem, through early endosome then late endosome, then lysosome
pinocytosis-
very tiny things; proteins, lipids. Goes through early, late, then lysosome
continuous process, rate depends on cell type
pinocytotic vesicle forms from clathrin coated pits in plasma mem
receptor mediated endocytosis- ligand binds to cell surface receptor, clathrin helps to form vesicle, clathrin coats vesicle
phagocytosis-
large things like bacteria; phagosome then to lysosome
feeding for lower single celled euks
multi celled orgs- used as a defense against invading microbes
requires surface receptors, triggered event
autophagy-
from ER, if we do not need certain organelles anymore, autophagosome forms then transported to lysosome
LDL Uptake
LDL- byproduct of fat transport, have ApoB protein
ApoB and LDL receptor bind
vesicle begins to form with help of clathrin coat
transported to early endosome→ late endosome→ lysosome
Disorders- LDL receptor missing, receptors do not associate with clathrin coat
Fate of cell surface receptors after endocytosis
recycling of receptor to same domain
receptor transported back to surface of membrane and pH will change→ receptor ready to bind to another LDL particle
degradation of receptor after endocytosis
in lysosome
transcytosis
any protein that is missent to basolateral side will be resent to apical membrane side
the vesicular transport of macromolecules from one side of a cell to the other
Slide Set 6: Microfilaments
The Cytoskeleton
Functions of cytoskeleton
cell shape, mvmt, and contraction
organelle mvmt and organization
cell division
intracellular org and vesicle mvmt
interacting with signaling pathways
basically like the bones of the cell
Components
Microfilaments
actin filaments, thinner
Microtubules
tubulin dimers, thicker
Intermediate filaments
various, diff proteins combined together
Cell signaling
signals tell cytoskeleton abt organization and mvmt of organelles as well as changes in cell shape, mvmt, and contraction
Actin Microfilaments
Functions
org of intracellular organelles and transport of vesicles (myosin)
intracellular mobility (bacteria)
cellular stability
cellular motility
muscle contraction
Lamellipodium
supported by growth of actin filaments, generates a protrusion structure to adhere to surface and move cell forward
Polymerization and Dynamics
1 actin filament= 2 strands
one + end (0.12 M), one - end (0.6)
g actin is monomer, microfilament polymer of actin
ATP binding cleft in actin structure
alpha, gamma, and beta actin: all associated with diff structures
G actin polymerization
g actin binds to f actin, elongating existing filament
can be added to + and - end, and leave from both sides
g actin dec to critical con→ polymer shrink
g actin inc above critical conc→ polymer inc in length
Actin Binding Proteins
Polymerization- Profilin and Thymosin B4
Profilin- promotes polymerization
Thymosin b4- blocks polymerization of ATP
Length- Cofilin, Gelsolin
Nucleation and branching- Arp2/3
Crosslinking- Filamin
Motor Proteins- myosin
stability/cap end of filaments- capz and tropomodulin
CapZ- caps at + end
Tropomodulin- caps at - end
org of filaments/muscle contraction, binds to side of filaments- nebulin
Actin based Motility
Formin - leads to assembly for long actin filaments
will form dimer structure
actin binds to structure and elongation commences
ARP2/3 complex can be used for polymerization to power motility, mediates branching
listeria monocytogenes uses actin polymerization to move through cells and from cell to cell→ hijack actin machinery and polymerize it to move around
ActA protein activates Arp2/3 to nucleate new filament assembly from preexisting filaments
filaments grow at + end until capped by Cap Z
actin recycled through cofilin, which enhances depolymerization at the - end of the filaments
this process propels bacterium forward
Toxins that perturb pool of actin monomers
Cytochalasin D- depolymerizes actin by blocking further addition of subunits
Latrunculin- inhibits g actin from adding to filament end
Jasplakinolide- stabilizes and binds actin dimers, lowers critical conc bar
Phalloidin- prevents actin filaments from depolymerizing by locking F subunits together
Actin also interacts with itself
types of lateral attachment of microfilaments to membranes
ankyrin- binds to Band 3 and then spectrin, forms network
band 4.1
Actin Motor Proteins
myosin
can bind to actin and help generate contraction in muscle cells
composed of heavy chains and light chains, diff myosin has diff amts of each
myosin heads can bind to ATP and actin
Myosin 1
small, single head
step size 10-14nm
works with membrane association and endocytosis
Myosin 2
dimer (2 heavy chain)
8nm step
bipolar filaments
works with contractions
Myosin 5
bigger, 2 heavy chains and more light chains
36nm step
responsible for organelle transport
Myosin mvmt process
ATP binds to head grp, head group not associated with actin yet
ATP hydrolyzed, head grp rotated into position to bind, head grp binds to actin
power stroke occurs, Pi released and myosin straightened, moving actin filament left
ADP released, the ATP bound and head grp released from actin
Step size vs neck length
is myosin step size/velocity proportional to neck length?
YES, velocity inc with inc neck length
contractile ring
myosin 2 takes a large part in forming when cells are splitting, myosin 1 is on outside of cells
Sarcomere (not protein, just structure of skeletal muscle)
vertical component is Z band, in between is A band, myosin in between actin filaments
actin end facing inside is - end
sarcoplasmic reticulum- specialized region of the ER, regulates and stores Ca (Ca helps muscle cells to contract)
Cap Z- binds to + end of actin
Tropomodulin- binds to - end of actin
Nebulin- binds to side of actin filaments
Titin- binds to myosin and Z disk proteins
Rho GTPases
membrane bound Rho proteins can bind effector proteins that cause changes in the actin cytoskeleton
dominant active rho- always keep making actin
Cdc42- filopodia formation
works at the front of cell, activates Rac
guys see a Rac and are activated
RacGTP- lamellipodia formation
leads to activation of Arp2/3 and Rho
RhoGTP- Stress fiber formation
leads to myosin 2 activation
Slide Set 7: Microtubules and Intermediate filaments
MIcrofilaments vs Microtubules vs Intermediate filaments
microfilaments
actin binds ATP
form rigid gels, networks, and bundles
tracks for myosin
contractile machinery and network at cell cortex
microtubules
tubulin binds GTP, rigid and not easily bent
trasks for kinesins and dyesins
organization for long range organelles
Intermediate filaments
great tensile strength, less dynamic, unpolarized
no motors
cell and tissue integrity
Microtubules
play a role in….
organization of organelles and transport of vesicles
mvmt of cilia and flagella
nerve cell, RBC, and flagellar structure
alignment and separation of chrom during mitosis
Tubulin
monomer of microtubules, alpha and beta make up monomer
Two populations of microtubules
Unstable short lived- assembles and disassembles rapidly
stable and long lived- remain polymerized for a long time (sperm flagella, RBC, nerve cells)
Polymerization and Structure of Microtubules
Structure
tubulin has alpha and beta parts
bind to 2 GTP
alpha T GTP is never hydrolyzed
beta T GTP can be hydrolyzed
one end is beta T exposed→ + end
one end is alpha T exposed → - end
microtubules made up of 13 protofilaments → singlet
can have doublets (cilia/flagella) and triplets (basal bodies and centrioles) as well
Polymerization
microtubules assembled from MTOC
MTOC-any structure used by cells to nucleate and organized microtubules
centrosome falls into this category
neg end of microtubules at MTOC
gamma tubulin ring nucleates microtubule assembly
Dynamics of Microtubules
Length over time: Assembly stage→ Catastrophe stage→ Disassembly stage→ Rescue Stage
Polymerization of tubulin into microtubules
protofilament first formed
alpha T first binds to protofilament, then beta T
sheet assembly
then form tube formation
GTP cap at top, GDP microtubule is the rest
GTP cap bc alpha and beta T carry GTP, addition of another Alpha and beta T will cause hydrolysis
end more smooth (assembly), - end more rough (disassembly)
Disassembly and reassembly of microtubules
cool to 4 deg, microtubule will disassemble
warm to 37 deg the microtubule will repolarize
Drugs that disrupt microtubule dynamics
colchincine- binds btwn alpha and beta T dimer so it cannot be used for polymerization, causes depolymerization
taxol- bind to side of tubules - stabilize the microtubule structure
Binding Proteins
MAPs
can stabilize microtubules, similar to taxol
side binding
MAP2- longer
Tau- shorter
+TIPS
can regulate + end of microtubules
Motors
Kinesins
ferry cargo around the cell
ferry towards + end
have light chain, bind to ATP for energy resource
bind to microtubule with head groups, bind to vesicle via kinesin receptor
hydrolyze ATP to drive mvmt
Kinesin 1 and 2- organelle, mRNA, and chromosome transport
Kinesin 5- bipolar structure, 2 head grps, can bind to 2 diff microtubules, microtubule sliding
Kinesin 13- can regulate microtubule end disassembly
Process
first head group, (leading head), no ATP, bound to microtubule
leading head then binds to ATP
conformational change induced, following head swings forwards
following head becomes leading head
new leading head releases ADP which it was originally bound to, and new following head hydrolyzes ATP to ADP and then process restarts
Dyneins
ferry cargo around the cell towards - end
Power stroke of dynein- ATP hydrolysis causes change in orientation of head→ mvmt of MT
dynactin- bind cargo, make dynein more processive
LIS1 protein- interact with ATPase domain of dynein to elongate power stroke
Intermediate filaments
heterogeneous
great tensile strength
no known motors use them as tracks
more stable than filaments or tubules
no intrinsic polarity
made up of protofilaments that can form diff structures
have N term and c term and head and tail end
keratin, lamin, vimentin
 Knowt
Knowt