
Biological Molecules
Carbohydrates
Carbohydrates are made out of Carbon (C), Hydrogen (H) and Oxygen (O)
triose = 3 carbon sugars (glyceraldehyde)
pentose = five carbon sugars (ribose, deoxyribose)
hexose = six carbon sugars (glucose, fructose, galactose)
Different positions of oxygen (H and OH) on the carbon chain are responsible for different properties of the molecule.
Properties of monosaccharides
simple sugars
small molecules
sweet
soluble in water
crystalline (forms crystals)
Functions of monosaccharides
a source of quick energy
Disaccharides
Each monosaccharide molecule is a monomer and can link together by glycosidic bonding.
A condensation reaction means that as two carbohydrate molecules bond together a water molecule is produced. The link formed between two glucose molecules is known as a glycosidic bond.
A glycosidic bond can also be broken down to release separate monomer units. Instead of water being given off a water molecule is needed to break down each glycosidic bond. This is called hydrolysis because water is needed to split up the bigger molecule.
Properties of disaccharides
sweet
soluble
2 monosaccharides joined together
Functions of disaccharides
transport in plants
preserve milk supply for off springs (humans + animals)
Polysaccharides
they consist of many monomer units linked by glycosidic bonds
they have many monomer units
polymers are chairs of sugar units
large molecules have important structural and strange roles
Starch (amylose) is a polymer of the sugar, glucose found in plants
Glycogen is a branched polymer of a-glucose found in humans
-glucose is stored in the liver
-it releases glucose for energy
-it is insoluble so it can remain in the cell
Properties of polysaccharides
macromolecules (large)
not sweet
insoluble
non crystalline (doesn’t form crystals)
Lipids
Fats, Oil, Steroids
They consist of the same elements as carbohydrates but their properties are different. They have high amounts of carbon and hydrogen, with a small amount of oxygen.
Fats and oils
Triglycerides
Triglycerides have twice as much fat as carbohydrates.
Fats and oils are formed when 3 fatty acid tails react with glycerol. A condensation reaction takes place (water is produced) and forms an ester bond.
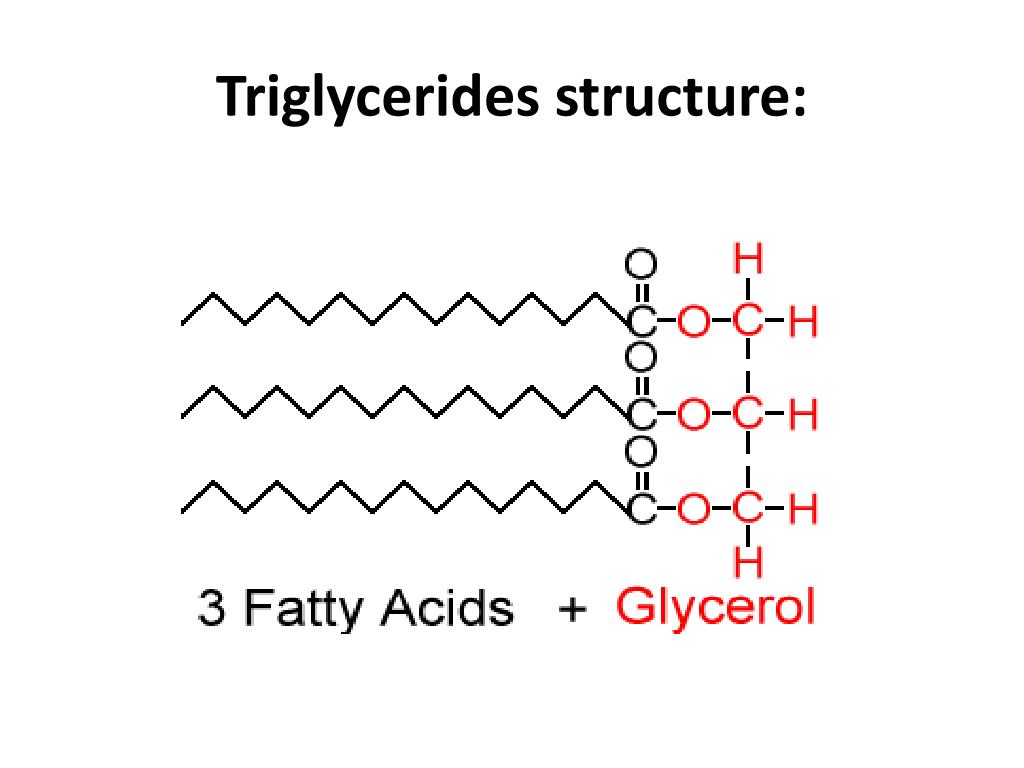
3 fatty acids + glycerol = fats and oils
Fatty Acids
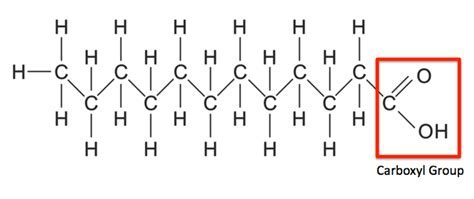
-long hydrocarbon tails
-carboxyl group at the end
-hydrocarbon tail makes up bulk of the fatty acid
Condensation reaction produces 3 water molecules and 3 ester bonds
Saturated vs Unsaturated fats
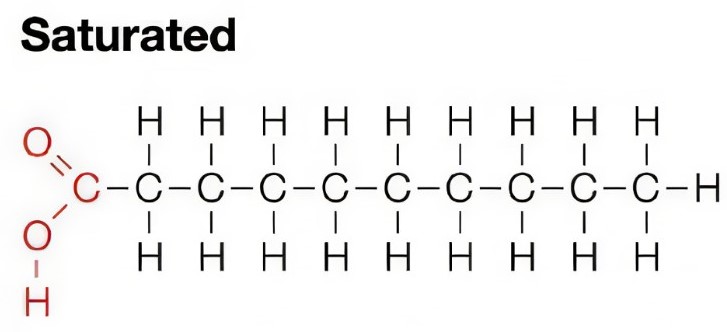
Saturated fats
-made from saturated fatty acids reacting with glycerol
-there are no double bonds between carbon atoms of the fatty acid tails
-the tails are straight as a result
-solid at room temperature
-melt at a high temperature
Unsaturated fats
-made from unsaturated fatty acids reacting with glycerol
-there are one or more double bonds between carbons
-this results in a bending of the fatty acid tail
-crooked tails prevent tight packaging
-liquid at room temperature

Lipids structure relates to function
-lipids are hydrophobic (don’t mix with water)
-this is due to the hydrocarbons making up to the fatty acid tails
-hydrocarbons are non polar so when lipids are placed into water, water would rather stick to itself than to the lipid.
Lipids and water don’t mix
Functions of lipids
The main function is energy storage
-one gram of fat stores twice as much energy of a gram of polysaccharide
-advantageous to animals that have to move around - unlike plants that have unlimited bulk without concern for mobility.
-cells that store fat = adipose cell
Cushioning - protects soft organs
Insulation - maintains body temperature
Electrical insulation and nerve cells
The main role is their function in cell membranes. To fulfil these functions a triglyceride fat is first converted into a phospholipid.
Phospholipids

glycerol + two fatty acid tails + one phosphate group
Phospholipid molecule is both hydrophobic and hydrophilic
-fatty acids are nonpolar and hydrophobic
-phosphate group is polar and hydrophilic
phosphoric acid replaces one of the fatty acids of the triglyceride. The phospholipid is a major component of cell membranes.
Steroids
Steroids are used to make hormones, vitamins and cholesterol
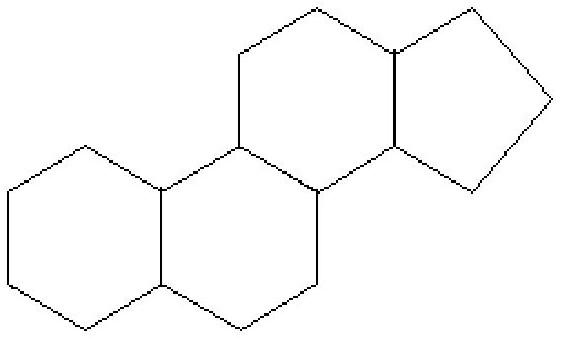
different functional groups are attracted to carbon rings.
Cholesterol
Cholesterol is a vital component of cell membranes.
Many hormones are steroids including oestrogen and testosterone, which are made from cholesterol
Protein
Proteins contain Carbon (C), Hydrogen (H), Oxygen (O) and Nitrogen (N)
They have a unique 3D shape which is critical to that protein’s function
Amino Acids

Carboxyl group - acidic properties COOH^-
Amino group - base properties NH2+
There are around 20 commonly found amino acids which differ by the r group.
Proteins are formed from polymers of amino acids called polypeptides.
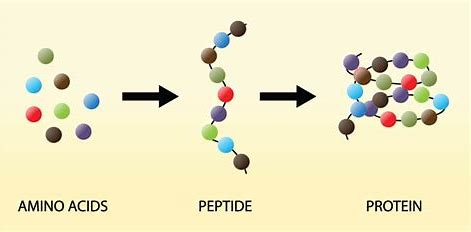
2 amino acids join with a peptide bond to form a dipeptide molecule.
A molecule of water is removed from two amino acids to form a peptide bond.
Condensation reaction as a water molecule is removed. Bonds are broken by hydrolysis.

-a functional protein is not just a polypeptide chain
-it is one or more polypeptide chains precisely twisted, folded and coiled into a uniquely shaped molecule
-it is the order of amino acids that determines the 3Dshape of a protein
-shape determines how the protein works
Primary Structure
 The order of the amino acids in the chain
The order of the amino acids in the chain
The 20 amino acids can be repeated and assembled in any order to form different sequences as part of protein synthesis. This sequence is controlled by DNA.
Secondary Structure
Polypeptides are held in position by hydrogen bonds. Hydrogen bonds are always between oxygen and hydrogen (O---H)

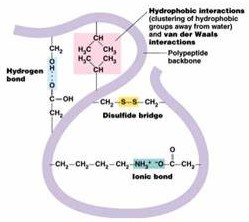
Tertiary Structure
This is when a polypeptide is folded into a precise shape. The polypeptide is held in ‘bends’ and ‘tusks’ in permanent shape by a range of of bonds including:
-disulphide bridges (sulphur - sulphur bonds)
-ionic bonds (due to the R group of amino acid)
-hydrogen bonds
This results in complex folding and twisting of the polypeptide
Quaternary Structure
Results when 2 or more polypeptide chains combine to make a functional protein.
e.g. collagen is a fibrous protein with 3 alpha helix chains twisted together
Protein Functions
Structural proteins - support
-keratin in hair and nails; Silk in cocoons/webs; collagen in connective tissue
Storage proteins - storage of amino acids
-Albumin in egg white; casein in milk#
Transport proteins - transport many substances across cell membranes or through the body
haemoglobin
Hormones - coordination of activities
-insulin (controls concentration of sugar in the blood)
Receptor proteins - receive chemical stimuli and respond
Contractile proteins - movement
Defensive proteins - protection against disease
Enzymes - speed up chemical reactions - thousands of different type of enzymes alone - each specifically designed for a particular chemical reaction
Enzymes
The Lock and Key Theory
The enzyme is the ‘lock’ and the substance is the ‘key’. To work one has to fit perfectly into the other, both are a fixed size and shape.
Induced Fit
The induced fit assumes that the substrate molecule can produce a change in the active site that enables the substrate to fit.
The substrates bind with the active site on the specific enzyme. The active site has a specific 3D shape as a result of enzyme's tertiary structure.
The active site changes shape around the substrate forming and ESC putting strain on the bonds in the substrate making it easier to make/ break bands as the activation energy is lowered.
The product (S) are released from the active site.
The active site returns to its original shape
Factors that affect enzyme activity
High temperatures and pH (away from the optimum) alter the three dimensional structure of enzyme molecules. Bonds involved in the tertiary structure may be broken and hence the configuration of the active site is altered, reducing the ability to form enzyme - substrate complexes and hence the reaction rate. High temperatures and extreme changes in pH cause a permanent change in an enzyme's structure, this is called denaturation.
Competitive inhibitor is similar shape to substrate so competes with enzyme for active site.
Non-competitive inhibitor is complementary to another site on the enzyme and distorts active site shape so substrate cannot bind to active site.
Biological Molecules
Carbohydrates
Carbohydrates are made out of Carbon (C), Hydrogen (H) and Oxygen (O)
triose = 3 carbon sugars (glyceraldehyde)
pentose = five carbon sugars (ribose, deoxyribose)
hexose = six carbon sugars (glucose, fructose, galactose)
Different positions of oxygen (H and OH) on the carbon chain are responsible for different properties of the molecule.
Properties of monosaccharides
simple sugars
small molecules
sweet
soluble in water
crystalline (forms crystals)
Functions of monosaccharides
a source of quick energy
Disaccharides
Each monosaccharide molecule is a monomer and can link together by glycosidic bonding.
A condensation reaction means that as two carbohydrate molecules bond together a water molecule is produced. The link formed between two glucose molecules is known as a glycosidic bond.
A glycosidic bond can also be broken down to release separate monomer units. Instead of water being given off a water molecule is needed to break down each glycosidic bond. This is called hydrolysis because water is needed to split up the bigger molecule.
Properties of disaccharides
sweet
soluble
2 monosaccharides joined together
Functions of disaccharides
transport in plants
preserve milk supply for off springs (humans + animals)
Polysaccharides
they consist of many monomer units linked by glycosidic bonds
they have many monomer units
polymers are chairs of sugar units
large molecules have important structural and strange roles
Starch (amylose) is a polymer of the sugar, glucose found in plants
Glycogen is a branched polymer of a-glucose found in humans
-glucose is stored in the liver
-it releases glucose for energy
-it is insoluble so it can remain in the cell
Properties of polysaccharides
macromolecules (large)
not sweet
insoluble
non crystalline (doesn’t form crystals)
Lipids
Fats, Oil, Steroids
They consist of the same elements as carbohydrates but their properties are different. They have high amounts of carbon and hydrogen, with a small amount of oxygen.
Fats and oils
Triglycerides
Triglycerides have twice as much fat as carbohydrates.
Fats and oils are formed when 3 fatty acid tails react with glycerol. A condensation reaction takes place (water is produced) and forms an ester bond.

3 fatty acids + glycerol = fats and oils
Fatty Acids

-long hydrocarbon tails
-carboxyl group at the end
-hydrocarbon tail makes up bulk of the fatty acid
Condensation reaction produces 3 water molecules and 3 ester bonds
Saturated vs Unsaturated fats
Saturated fats
-made from saturated fatty acids reacting with glycerol
-there are no double bonds between carbon atoms of the fatty acid tails
-the tails are straight as a result
-solid at room temperature
-melt at a high temperature

Unsaturated fats
-made from unsaturated fatty acids reacting with glycerol
-there are one or more double bonds between carbons
-this results in a bending of the fatty acid tail
-crooked tails prevent tight packaging
-liquid at room temperature

Lipids structure relates to function
-lipids are hydrophobic (don’t mix with water)
-this is due to the hydrocarbons making up to the fatty acid tails
-hydrocarbons are non polar so when lipids are placed into water, water would rather stick to itself than to the lipid.
Lipids and water don’t mix
Functions of lipids
The main function is energy storage
-one gram of fat stores twice as much energy of a gram of polysaccharide
-advantageous to animals that have to move around - unlike plants that have unlimited bulk without concern for mobility.
-cells that store fat = adipose cell
Cushioning - protects soft organs
Insulation - maintains body temperature
Electrical insulation and nerve cells
The main role is their function in cell membranes. To fulfil these functions a triglyceride fat is first converted into a phospholipid.
Phospholipids

glycerol + two fatty acid tails + one phosphate group
Phospholipid molecule is both hydrophobic and hydrophilic
-fatty acids are nonpolar and hydrophobic
-phosphate group is polar and hydrophilic
phosphoric acid replaces one of the fatty acids of the triglyceride. The phospholipid is a major component of cell membranes.
Steroids
Steroids are used to make hormones, vitamins and cholesterol

different functional groups are attracted to carbon rings.
Cholesterol
Cholesterol is a vital component of cell membranes.
Many hormones are steroids including oestrogen and testosterone, which are made from cholesterol
Protein
Proteins contain Carbon (C), Hydrogen (H), Oxygen (O) and Nitrogen (N)
They have a unique 3D shape which is critical to that protein’s function
Amino Acids

Carboxyl group - acidic properties COOH^-
Amino group - base properties NH2+
There are around 20 commonly found amino acids which differ by the r group.
Proteins are formed from polymers of amino acids called polypeptides.

2 amino acids join with a peptide bond to form a dipeptide molecule.
A molecule of water is removed from two amino acids to form a peptide bond.
Condensation reaction as a water molecule is removed. Bonds are broken by hydrolysis.

-a functional protein is not just a polypeptide chain
-it is one or more polypeptide chains precisely twisted, folded and coiled into a uniquely shaped molecule
-it is the order of amino acids that determines the 3Dshape of a protein
-shape determines how the protein works
Primary Structure
 The order of the amino acids in the chain
The order of the amino acids in the chain
The 20 amino acids can be repeated and assembled in any order to form different sequences as part of protein synthesis. This sequence is controlled by DNA.
Secondary Structure
Polypeptides are held in position by hydrogen bonds. Hydrogen bonds are always between oxygen and hydrogen (O---H)

Tertiary Structure
This is when a polypeptide is folded into a precise shape. The polypeptide is held in ‘bends’ and ‘tusks’ in permanent shape by a range of of bonds including:
-disulphide bridges (sulphur - sulphur bonds)
-ionic bonds (due to the R group of amino acid)
-hydrogen bonds
This results in complex folding and twisting of the polypeptide

Quaternary Structure
Results when 2 or more polypeptide chains combine to make a functional protein.
e.g. collagen is a fibrous protein with 3 alpha helix chains twisted together
Protein Functions
Structural proteins - support
-keratin in hair and nails; Silk in cocoons/webs; collagen in connective tissue
Storage proteins - storage of amino acids
-Albumin in egg white; casein in milk#
Transport proteins - transport many substances across cell membranes or through the body
haemoglobin
Hormones - coordination of activities
-insulin (controls concentration of sugar in the blood)
Receptor proteins - receive chemical stimuli and respond
Contractile proteins - movement
Defensive proteins - protection against disease
Enzymes - speed up chemical reactions - thousands of different type of enzymes alone - each specifically designed for a particular chemical reaction
Enzymes
The Lock and Key Theory
The enzyme is the ‘lock’ and the substance is the ‘key’. To work one has to fit perfectly into the other, both are a fixed size and shape.
Induced Fit
The induced fit assumes that the substrate molecule can produce a change in the active site that enables the substrate to fit.
The substrates bind with the active site on the specific enzyme. The active site has a specific 3D shape as a result of enzyme's tertiary structure.
The active site changes shape around the substrate forming and ESC putting strain on the bonds in the substrate making it easier to make/ break bands as the activation energy is lowered.
The product (S) are released from the active site.
The active site returns to its original shape
Factors that affect enzyme activity
High temperatures and pH (away from the optimum) alter the three dimensional structure of enzyme molecules. Bonds involved in the tertiary structure may be broken and hence the configuration of the active site is altered, reducing the ability to form enzyme - substrate complexes and hence the reaction rate. High temperatures and extreme changes in pH cause a permanent change in an enzyme's structure, this is called denaturation.
Competitive inhibitor is similar shape to substrate so competes with enzyme for active site.
Non-competitive inhibitor is complementary to another site on the enzyme and distorts active site shape so substrate cannot bind to active site.
 Knowt
Knowt