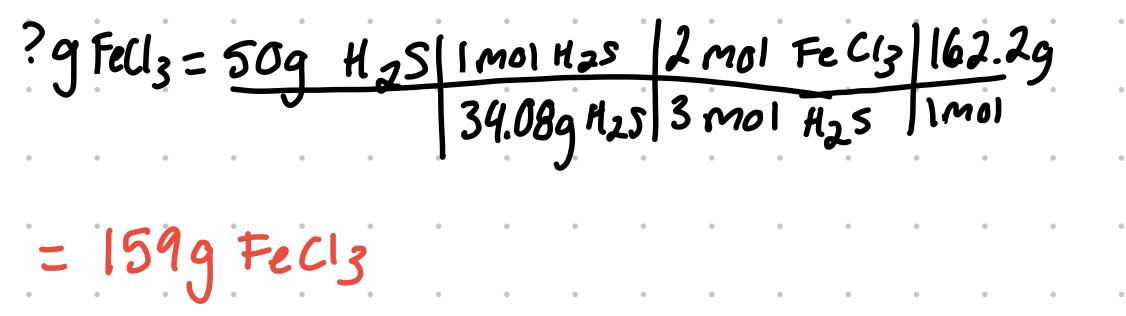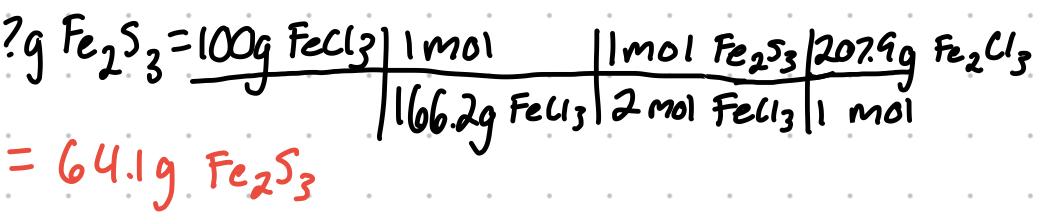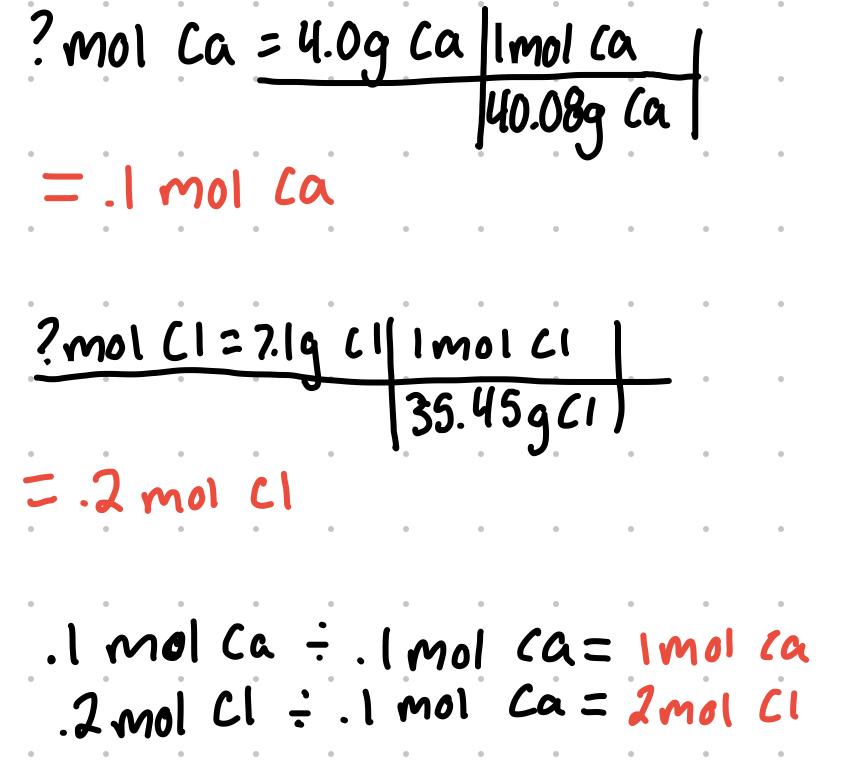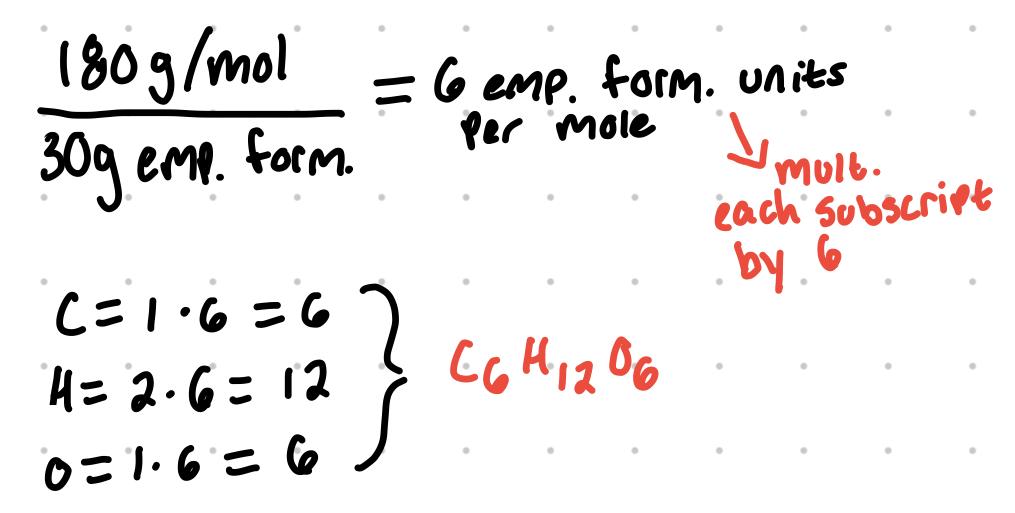Chapter 5: Stoichiometry
Stoichiometry is the quantitative relationship between different values and units
Dimensional Analysis is the primary stoichiometry method
Conversion Calculations Using the Dimensional Analysis Method
Defining Conversion Factions
The conversion factor in dimensional analysis converts values to different units
Example: when go from yards to inches, the conversion factor is 36 inches to one yard. Thus, multiple 2 yards by 36 inches to get 72 inches
Using Conversion Factors
Conversion problems must 2 pieces of info
The number and the units that need to be converted
The units of the answer
Example: “? answer units = xxx given units”
When converting, the units that are NOT for the answer will cancel out and only the answer units will remain
Conversion of Metric Units
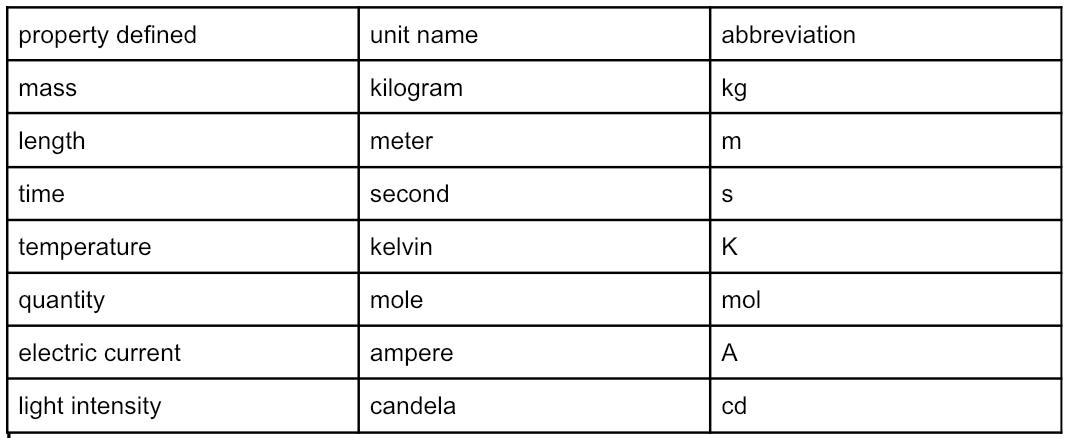
Base units of SI (international system)
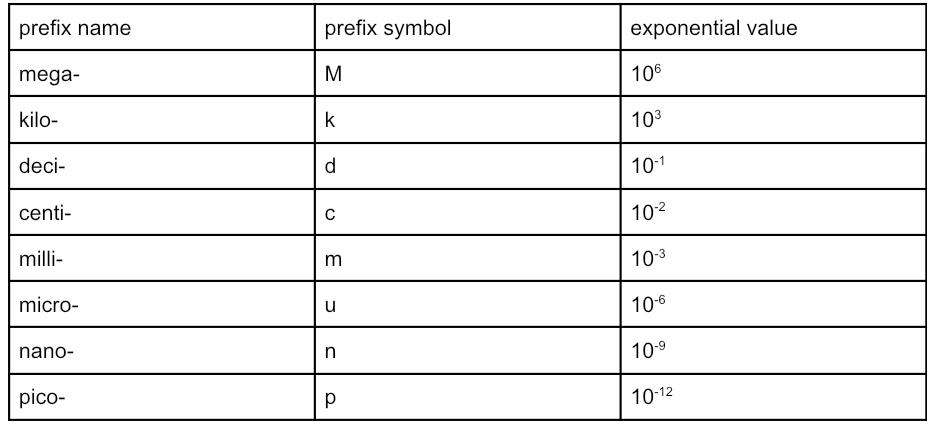
metric prefixes are added to multiple base units
Chemical Equalities and Equivalences
The Mole and Avogadro’s Number
mole is 6.02 × 10^23 units of a chemical substance
1 mole of CH4 is 6.02 × 10^23 molecules
1 mole argon atoms is 6.02 × 10^23 atoms
6.02 × 10^23 is Avogadro’s Constant
symbol n in mathematical equations
Molar Mass
relative atomic mass has no units and indicates the mass of one element
gram-atomic mass
1 mole of an element = gram-atomic mass of element
gram-molar mass
1 mole of compound = gram-molar mass of that compound
1 mole argon = 39.948 grams of argon
1 mole CH4 = 16.043 grams of CH4
Conversion Factors from Chemical Formulas
Empirical formula is the simplest ratio of atoms
Chemical formula is true ratio
1 molecule C12H22O11 = 12 atoms of carbon, 22 atoms of hydrogen, 11 atoms of oxygen
Usually referred to in mole value vs atom value
12 mol carbon, 22 mol hydrogen, 11 mol oxygen
Conversion Factors from Balanced Chemical Equations
A chemical equation can be used at a relationship and conversion factor
for benzene combustion, 2C6H6 + 15 O2 → 12CO2 + 6H20
2 mol C6H6, 15 mol O2 is equal to
12 mol CO2 and 6 mol H2O
Concentration and Density as Conversion Factors
Concentration unit is Molarity, M
number of moles dissolved in one liter of solution
if mL of solution is given, it must be converted to L
if density is given, cm^3 can be converted to .741g
1mL = cm^3
Other Conversion Factors
under standard conditions (STP), 1 mole of a gas occupies 22.4 liters
STP is 1 atmosphere of pressure and 0 degrees celsius
The Conversion Sequence /h2
When converting from substance A to substance B, A must go to moles then go to mole of substance B, then convert to the final units
Limiting-Reactant Calculations
When chemicals are mixed and a reaction occurs, the first reactant completely used up (which causes the reaction to end), that is the limiting reactant or limiting reagent
For the problem, determine the limiting reactant if 100. g of FeCl3 are reacted with 50.0 g of H2S: 2FeCl3 + 3H2S → Fe2S3 + 6HCl
since 159g is required of FeCl3 to fully react with 50g H2S, the FeCl3 is the limiting reactant since there is not enough. If the answer was less than the given 100g, the H2S would be limiting reactant since there would be more FeCl3 available to use.
Determining the Theoretical Yield
Theoretical yield is the maximum amount of product that would be formed in a reaction. It is theoretical because events in a lab could cause it to decrease
What is the theoretical yield of Fe2S3 that can be obtained from 100g of FeCl3 and 50g of H2S
Titrations
Titrations are used to add one substance to another in a controlled manner. Usually an acid added to a base with a goal of changing the pH to a certain threshold. Uses a color indicator occasionally
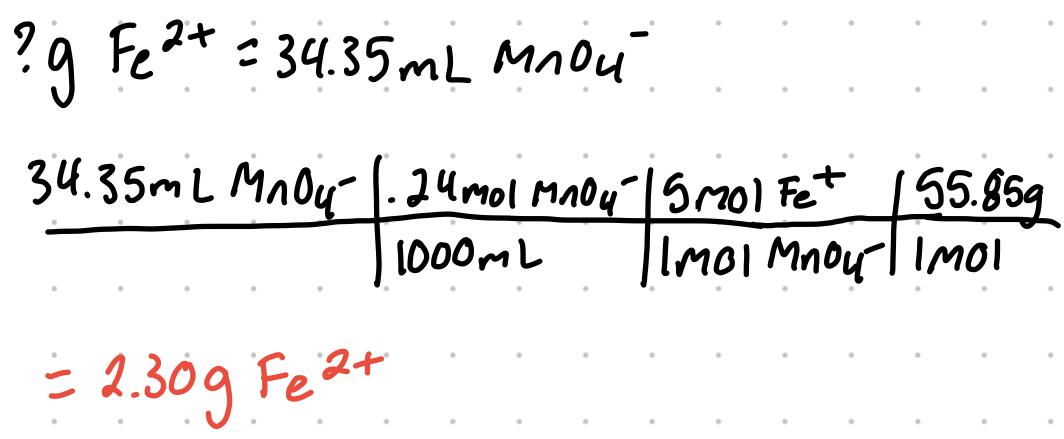
It takes 34.35 mL of a 0.240 M solution of KMnO4 (0.240M MnO4-) to titrate an unknown sample of Fe2+ to its endpoint. How many grams of Fe2+ are in the sample?
Percent Composition
Percent composition is how much of an element is present in a polyatomic ion. Percent composition is how much of the mass of the element is in 100 grams of a chemical compound.
What is the percentage of Fe, O, H and the OH- polyatomic ion in Fe(OH)3?
Empirical Formulas
If given a chemical formula and atomic masses of elements, percent composition can be calculated.
With percent composition and atomic masses given, the empirical formula (simplest ratio of elements in a compound) can be determined.
Benzene, C6H6, has an empirical formula of CH.
Sugar glucose, C6H12,06, has an empirical formula of CH2O
To find empirical formula…
Calculate the number of moles of each element in the compound
Divide the number of moles calucated of each element by the smallest number of moles
If the previous step does not give a whole number or a decimal within .1 of a whole number, multiply each element by a fraction to get a whole number.
Example
What is the empirical formula of a compound that contains 4.0g of calcium and 7.1 g of chlorine?
The empirical formula has 1 mol Ca and 2 mol Cl. CaCl2
Molecular Formulas
The empirical formula is the simplest ratio. The molecular formula is the actual formula.
If the empirical formula is known or determined, the molar mass of the compound can be used to determine the molecular formula
A compound has an empirical formula of CH2O and has a molar mass of 180 g/mol. What is the molecular formula of the compound?
Chapter 5: Stoichiometry
Stoichiometry is the quantitative relationship between different values and units
Dimensional Analysis is the primary stoichiometry method
Conversion Calculations Using the Dimensional Analysis Method
Defining Conversion Factions
The conversion factor in dimensional analysis converts values to different units
Example: when go from yards to inches, the conversion factor is 36 inches to one yard. Thus, multiple 2 yards by 36 inches to get 72 inches
Using Conversion Factors
Conversion problems must 2 pieces of info
The number and the units that need to be converted
The units of the answer
Example: “? answer units = xxx given units”
When converting, the units that are NOT for the answer will cancel out and only the answer units will remain
Conversion of Metric Units
Base units of SI (international system)
metric prefixes are added to multiple base units
Chemical Equalities and Equivalences
The Mole and Avogadro’s Number
mole is 6.02 × 10^23 units of a chemical substance
1 mole of CH4 is 6.02 × 10^23 molecules
1 mole argon atoms is 6.02 × 10^23 atoms
6.02 × 10^23 is Avogadro’s Constant
symbol n in mathematical equations
Molar Mass
relative atomic mass has no units and indicates the mass of one element
gram-atomic mass
1 mole of an element = gram-atomic mass of element
gram-molar mass
1 mole of compound = gram-molar mass of that compound
1 mole argon = 39.948 grams of argon
1 mole CH4 = 16.043 grams of CH4
Conversion Factors from Chemical Formulas
Empirical formula is the simplest ratio of atoms
Chemical formula is true ratio
1 molecule C12H22O11 = 12 atoms of carbon, 22 atoms of hydrogen, 11 atoms of oxygen
Usually referred to in mole value vs atom value
12 mol carbon, 22 mol hydrogen, 11 mol oxygen
Conversion Factors from Balanced Chemical Equations
A chemical equation can be used at a relationship and conversion factor
for benzene combustion, 2C6H6 + 15 O2 → 12CO2 + 6H20
2 mol C6H6, 15 mol O2 is equal to
12 mol CO2 and 6 mol H2O
Concentration and Density as Conversion Factors
Concentration unit is Molarity, M
number of moles dissolved in one liter of solution
if mL of solution is given, it must be converted to L
if density is given, cm^3 can be converted to .741g
1mL = cm^3
Other Conversion Factors
under standard conditions (STP), 1 mole of a gas occupies 22.4 liters
STP is 1 atmosphere of pressure and 0 degrees celsius
The Conversion Sequence /h2
When converting from substance A to substance B, A must go to moles then go to mole of substance B, then convert to the final units
Limiting-Reactant Calculations
When chemicals are mixed and a reaction occurs, the first reactant completely used up (which causes the reaction to end), that is the limiting reactant or limiting reagent
For the problem, determine the limiting reactant if 100. g of FeCl3 are reacted with 50.0 g of H2S: 2FeCl3 + 3H2S → Fe2S3 + 6HCl
since 159g is required of FeCl3 to fully react with 50g H2S, the FeCl3 is the limiting reactant since there is not enough. If the answer was less than the given 100g, the H2S would be limiting reactant since there would be more FeCl3 available to use.
Determining the Theoretical Yield
Theoretical yield is the maximum amount of product that would be formed in a reaction. It is theoretical because events in a lab could cause it to decrease
What is the theoretical yield of Fe2S3 that can be obtained from 100g of FeCl3 and 50g of H2S
Titrations
Titrations are used to add one substance to another in a controlled manner. Usually an acid added to a base with a goal of changing the pH to a certain threshold. Uses a color indicator occasionally
It takes 34.35 mL of a 0.240 M solution of KMnO4 (0.240M MnO4-) to titrate an unknown sample of Fe2+ to its endpoint. How many grams of Fe2+ are in the sample?
Percent Composition
Percent composition is how much of an element is present in a polyatomic ion. Percent composition is how much of the mass of the element is in 100 grams of a chemical compound.
What is the percentage of Fe, O, H and the OH- polyatomic ion in Fe(OH)3?
Empirical Formulas
If given a chemical formula and atomic masses of elements, percent composition can be calculated.
With percent composition and atomic masses given, the empirical formula (simplest ratio of elements in a compound) can be determined.
Benzene, C6H6, has an empirical formula of CH.
Sugar glucose, C6H12,06, has an empirical formula of CH2O
To find empirical formula…
Calculate the number of moles of each element in the compound
Divide the number of moles calucated of each element by the smallest number of moles
If the previous step does not give a whole number or a decimal within .1 of a whole number, multiply each element by a fraction to get a whole number.
Example
What is the empirical formula of a compound that contains 4.0g of calcium and 7.1 g of chlorine?
The empirical formula has 1 mol Ca and 2 mol Cl. CaCl2
Molecular Formulas
The empirical formula is the simplest ratio. The molecular formula is the actual formula.
If the empirical formula is known or determined, the molar mass of the compound can be used to determine the molecular formula
A compound has an empirical formula of CH2O and has a molar mass of 180 g/mol. What is the molecular formula of the compound?
 Knowt
Knowt