
Unit 4 - States & Changes of Matter
Gases
Kinetic-Molecular Theory
Gases are a large number of constantly and randomly moving particles
Most of the volume of a gas is empty space
There is no force of attraction/repulsion between particles
All collisions are perfectly elastic
No energy is gained/loss during collisions
Kinetic Energy - The energy of movement [of particles]
Average kinetic energy is directly proportional to temperature
KE = (1/2)mv^2
Ideal Gas - A theoretical gas composed of randomly moving particles that don’t interact with each other
Describes the behavior of most gases under common conditions
Fits the description of the Kinetic-Molecular Theory
Real gases deviating from this model under extreme conditions
Properties of Gases
Compressibility
Change in pressure → change in volume
No fixed shape/volume
Expands to fit container
Property Relationships
Pressure & Volume have an inverse relationship
Volume and Number of Atoms(# of moles) have a direct relationship
Pressure and Temperature have a direct relationship
Collision with container walls cause pressure
More/harder collisions = more pressure
Pressure = Force / Area
Volume and Temperature have a direct relationship
Pressure and Number of Atoms(# of moles) have a direct relationship
Diffusion - Movement of particles from high concentration to low concentration
Effusion - Movement of gas through a smaller opening into a larger volume
Graham’s Law - Rate of effusion is inversely proportional to the square root of molar mass
Liquids
Kinetic energy of individual particles is similar to that of the intermolecular attraction between them
Properties
More dense than gases
Have a fixed volume
Particles aren’t fixed in place
Can flow freely
Viscosity - The thickness / resistance to flow of a liquid
Directly related to intermolecular force
Directly related to size of molecules
Inversely related to temperature
Takes the shape of their container
Surface Tension - The tendency for a liquid to resist penetration
Directly related to intermolecular force
Surfactant - chemical compounds that can decrease surface tension
Incompressible
Can be used to transmit force, i.e hydraulics
Dissolvability - When a solid, liquid, or gas becomes integrated into a host liquid
Dissolved particles are dispersed evenly throughout the liquid
Miscible Liquids - liquids that are able to dissolve into each other
Immiscible Liquids - liquids that are not able to dissolve into each other
Intermolecular Forces
Caused by uneven electron distribution
Affects interactions between particles
The stronger the force, the more kinetic energy particles need to move
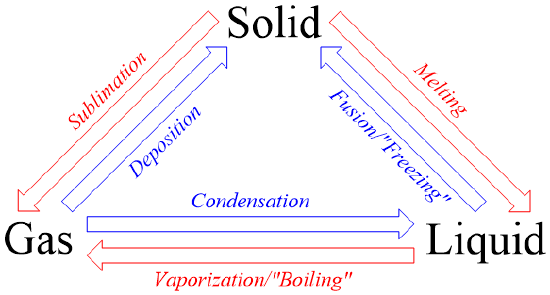
Condensation - Gas → Liquid
Caused by intermolecular force > kinetic energy, meaning particles are pulled in towards each other
Evaporation/boiling - Liquid → Gas
*Evaporation is when small amounts of particles randomly gain enough energy, boiling is when the entire substance as a whole gains enough energy to change
Caused by kinetic energy > intermolecular force, meaning particles are pulled away from each other
Boiling point has a direct correlation w/ boiling points
Higher intermolecular force → higher boiling point
i.e ionic and polar covalent compounds have higher boiling points like non-polar covalent compounds
Freezing - Liquid → Solid
Caused by intermolecular force > kinetic energy
Melting - Solid → Liquid
Caused by kinetic energy > intermolecular force
Solids and Plasmas
Solids
Properties
Low energy
Rigid structure
Molecules vibrates instead of move
Fixed shape & volume
Crystal - A solid whos components make up a highly ordered microscopic structure
Long Range Order - A property of crystals where their atomic particles show a periodic (recurring) pattern or shape
Lattice - A regular arrangement of atoms, molecules, etc
Incompressible
Amorphous Solids - Solids with particles arranged in non-uniform patterns
Can be caused by rapid cooling such that particles do not have time to fully arrange into a crystalline structure
Lack of long range order
Compressible
No definitive properties like boiling points due to changing pattern of molecules throughout
Molecules can shift & move past each other over time
Plasmas
Properties
Composed of ionized (high-energy) particles
EXTREMELY HOT (high temperature)
Conducts electricity
Compressible
No definite volume/shape
Examples: Lightning, Stars, Auroras, Fluorescent Lights, Ion Thrusters, Arc Welders, Plasma Displays (plasma TVs), Plasma Balls, etc
Thermal Equilibrium - Temperature is equal to its surroundings
Plasma can be “cold“ when:
Their electrons break off from their nucleuses and move extremely quickly, dissipating the energy quickly
The energy in the electrons gets converted to light
Only a small percentage of the overall substance is ionized into a plasma
Comparisons
Conduct Electricity - Plasmas always conduct electricity, only some solids do
Density - Plasmas have low density, solids have high density
Shape & Volume - Solids have fixed shape and volume, plasmas don’t
Kinetic Energy - Plasmas have high kinetic energy, solids have low kinetic energy
Composition - Plasmas are made of electrons and cations, solids are made of neutral particles or cation/anion pairs
Phase Changes
Most of the phase changes are covered previously, so the only information here is going to be non-covered vocab and concepts
Vapor Pressure - The pressure exerted by the gas in equilibrium with a liquid
Changes based on altitude; higher pressure → harder to boil
Higher vapor pressure → more likely to evaporate
Properties of Water
Water = H2O
Has a total of 8 valence electrons; stable
There are single bonds between the oxygen and each hydrogen, and two pairs of non-bonded electrons on the other side of the oxygen atom
“Tetrahedral“ electron-domain geometry
“bent“ molecular geometry
Bond Angle of 104.5*
Properties
Oxygen --- Hydrogen bonds are highly polar → water is a polar molecule
Oxygen is partial negative, hydrogens are partial positive
Allows for hydrogen bonds
Strong solvent
Like dissolves like → Water mostly dissolves ionic and polar covalent compounds
Process of dissolving
Dissociation - Water breaks an ionic compound into cations and anions
Hydration - Water surrounds “broken apart“ substances
Adhesion/Cohesion - Intermolecular forces; polar “stick“ to polar
Adhesion is with other molecules, cohesion is with itself
Strong surface tension
High specific heat capacity
Uses

Unit 4 - States & Changes of Matter
Gases
Kinetic-Molecular Theory
Gases are a large number of constantly and randomly moving particles
Most of the volume of a gas is empty space
There is no force of attraction/repulsion between particles
All collisions are perfectly elastic
No energy is gained/loss during collisions
Kinetic Energy - The energy of movement [of particles]
Average kinetic energy is directly proportional to temperature
KE = (1/2)mv^2
Ideal Gas - A theoretical gas composed of randomly moving particles that don’t interact with each other
Describes the behavior of most gases under common conditions
Fits the description of the Kinetic-Molecular Theory
Real gases deviating from this model under extreme conditions
Properties of Gases
Compressibility
Change in pressure → change in volume
No fixed shape/volume
Expands to fit container
Property Relationships
Pressure & Volume have an inverse relationship
Volume and Number of Atoms(# of moles) have a direct relationship
Pressure and Temperature have a direct relationship
Collision with container walls cause pressure
More/harder collisions = more pressure
Pressure = Force / Area
Volume and Temperature have a direct relationship
Pressure and Number of Atoms(# of moles) have a direct relationship
Diffusion - Movement of particles from high concentration to low concentration
Effusion - Movement of gas through a smaller opening into a larger volume
Graham’s Law - Rate of effusion is inversely proportional to the square root of molar mass
Liquids
Kinetic energy of individual particles is similar to that of the intermolecular attraction between them
Properties
More dense than gases
Have a fixed volume
Particles aren’t fixed in place
Can flow freely
Viscosity - The thickness / resistance to flow of a liquid
Directly related to intermolecular force
Directly related to size of molecules
Inversely related to temperature
Takes the shape of their container
Surface Tension - The tendency for a liquid to resist penetration
Directly related to intermolecular force
Surfactant - chemical compounds that can decrease surface tension
Incompressible
Can be used to transmit force, i.e hydraulics
Dissolvability - When a solid, liquid, or gas becomes integrated into a host liquid
Dissolved particles are dispersed evenly throughout the liquid
Miscible Liquids - liquids that are able to dissolve into each other
Immiscible Liquids - liquids that are not able to dissolve into each other
Intermolecular Forces
Caused by uneven electron distribution
Affects interactions between particles
The stronger the force, the more kinetic energy particles need to move
Condensation - Gas → Liquid
Caused by intermolecular force > kinetic energy, meaning particles are pulled in towards each other
Evaporation/boiling - Liquid → Gas
*Evaporation is when small amounts of particles randomly gain enough energy, boiling is when the entire substance as a whole gains enough energy to change
Caused by kinetic energy > intermolecular force, meaning particles are pulled away from each other
Boiling point has a direct correlation w/ boiling points
Higher intermolecular force → higher boiling point
i.e ionic and polar covalent compounds have higher boiling points like non-polar covalent compounds
Freezing - Liquid → Solid
Caused by intermolecular force > kinetic energy
Melting - Solid → Liquid
Caused by kinetic energy > intermolecular force
Solids and Plasmas
Solids
Properties
Low energy
Rigid structure
Molecules vibrates instead of move
Fixed shape & volume
Crystal - A solid whos components make up a highly ordered microscopic structure
Long Range Order - A property of crystals where their atomic particles show a periodic (recurring) pattern or shape
Lattice - A regular arrangement of atoms, molecules, etc
Incompressible
Amorphous Solids - Solids with particles arranged in non-uniform patterns
Can be caused by rapid cooling such that particles do not have time to fully arrange into a crystalline structure
Lack of long range order
Compressible
No definitive properties like boiling points due to changing pattern of molecules throughout
Molecules can shift & move past each other over time
Plasmas
Properties
Composed of ionized (high-energy) particles
EXTREMELY HOT (high temperature)
Conducts electricity
Compressible
No definite volume/shape
Examples: Lightning, Stars, Auroras, Fluorescent Lights, Ion Thrusters, Arc Welders, Plasma Displays (plasma TVs), Plasma Balls, etc
Thermal Equilibrium - Temperature is equal to its surroundings
Plasma can be “cold“ when:
Their electrons break off from their nucleuses and move extremely quickly, dissipating the energy quickly
The energy in the electrons gets converted to light
Only a small percentage of the overall substance is ionized into a plasma
Comparisons
Conduct Electricity - Plasmas always conduct electricity, only some solids do
Density - Plasmas have low density, solids have high density
Shape & Volume - Solids have fixed shape and volume, plasmas don’t
Kinetic Energy - Plasmas have high kinetic energy, solids have low kinetic energy
Composition - Plasmas are made of electrons and cations, solids are made of neutral particles or cation/anion pairs
Phase Changes
Most of the phase changes are covered previously, so the only information here is going to be non-covered vocab and concepts
Vapor Pressure - The pressure exerted by the gas in equilibrium with a liquid
Changes based on altitude; higher pressure → harder to boil
Higher vapor pressure → more likely to evaporate

Properties of Water
Water = H2O
Has a total of 8 valence electrons; stable
There are single bonds between the oxygen and each hydrogen, and two pairs of non-bonded electrons on the other side of the oxygen atom
“Tetrahedral“ electron-domain geometry
“bent“ molecular geometry
Bond Angle of 104.5*
Properties
Oxygen --- Hydrogen bonds are highly polar → water is a polar molecule
Oxygen is partial negative, hydrogens are partial positive
Allows for hydrogen bonds
Strong solvent
Like dissolves like → Water mostly dissolves ionic and polar covalent compounds
Process of dissolving
Dissociation - Water breaks an ionic compound into cations and anions
Hydration - Water surrounds “broken apart“ substances
Adhesion/Cohesion - Intermolecular forces; polar “stick“ to polar
Adhesion is with other molecules, cohesion is with itself
Strong surface tension
High specific heat capacity
Uses

 Knowt
Knowt