
Pharmacology test #3
18 Pain, Anesthesia and Drug Treatment
Physiology of Pain
Peripheral Nervous System: detects temperature, pressure, touch, pain
Sensation: Heat, cold, pressure, or chemical stimulus to sensory receptors and nerve endings in the peripheral nervous system
Pain Receptors: Perceive the sensation of pain when stimulated
Pain: as physically or emotionally sensed discomfort that is associated with acute tissue damage or a sensory system malfunction
Pain receptors, known as nociceptors, are activated when a stimulus could damage tissue.
Pain warns of damage or the presence of disease.
Pain is a normal part of healing
Pain Management
Pain
a normal physiologic response to stimuli usually associated with tissue damage
alerts the body to injury or inflammation
Inflammation: causes a cascade of events that untimely forms prostaglandins
Prostaglandins
chemical triggers for pain
causes local redness and swelling
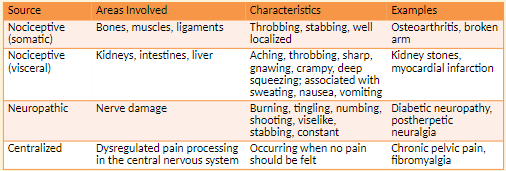
Types of Pain
Somatic Pain: originates from injury to body tissue
ex. include post-surgical incision pain and bone pain from cancer metastases
Throbbing, stabbing, well localized
Narcotics, NSAIDs, nerve blockers
Visceral Pain: originates from problems with internal organs
Signals not received from these organs by sensory nerves
Symptoms include nausea, vomiting, and sweating, Aching, throbbing, sharp, gnawing, crampy, deep squeezing; associated with sweating, nausea, vomiting
Narcotics, NSAIDs, nerve blockers, antiemetics
Neuropathic Pain: from damage of nerve tissue
Symptoms include a tingling, burning, or stabbing pain in area of injury; pain frequently radiates.
Burning, aching, numbing, tingling, viselike, knifelike, constant
Antidepressants, anticonvulsants
Sympathetic-mediated pain; centralized: Patient feels pain when there is no obvious stimulus
Example is phantom-limb pain in which pain is felt in a limb that is no longer there.
Occurring when no pain should be felt
Nerve blockers
Timing of Pain
Acute Pain
Associated with trauma or surgery
Occurs within 6 weeks of a pain-inducing event
Has a beginning and an end, warns of a problem
Pain that lasts less than six months
Ranges from mild to severe
Sympathetic nervous system response includes increased pulse, blood pressure, and breathing rate; tensing of muscles; dilation of pupils
Chronic nonmalignant Pain
Cause may or may not be diagnosed.
Lasts > 3-6 months, may respond poorly to treatment
Does not cease when an illness or injury is healed
Compensatory response includes feelings of hopelessness, lack of facial expression, isolation, fatigue, anger, physical inactivity.
Chronic Malignant Pain
accompanies malignant disease
Increases in severity as diseases progresses
Response to Pain
Background pain: describes a constant level of pain.
Breakthrough pain:describes when pain of greater intensity occurs intermittently for no particular reason.
Provoked pain: describes pain that is more intense than background pain but has a clear cause.
Pain Assessment Scales
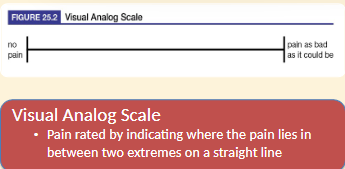
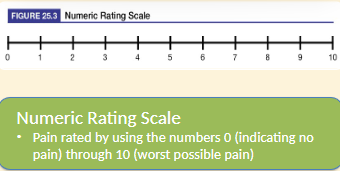
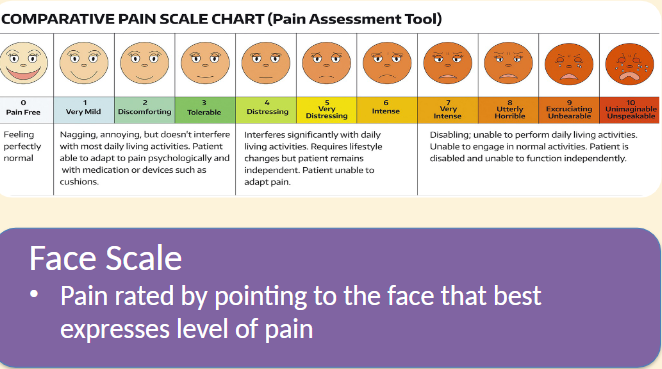
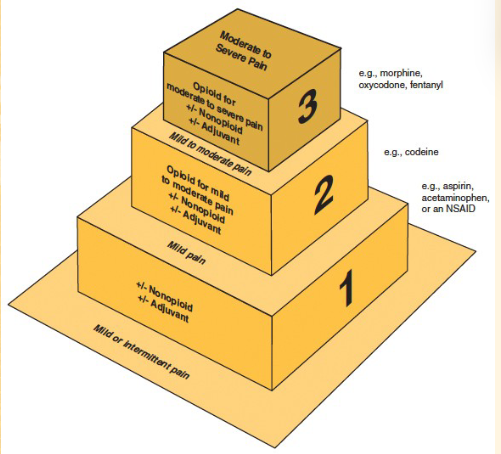
Categories of Drugs for Pain:
Narcotics- severe pain
non-narcotics- moderate/mind pain
NSAIDs- moderate/mild pain
The “Ideal” Analgesic
would…
provide maximum pain relief
produce no side effects
cause no dependence or addiction
Mild-to-moderate Pain Disorders
mild pain: described as 1 to 4 on a numeric rating scale
moderate pain: a 5-6 on a numeric rating scale
causes of mild-to-moderate pain: a variety of disorders including headaches
Treatment: non-narcotic Angelic
work independently of opioid receptors
mild-to-moderate trying to treat without narcotics
Non-narcotic Analgesics: Acetaminophen
liver toxicity
Doses > 4 g per day
Chronic use at daily maximum doses
Permanent liver damage with intentional overdose
Alcohol Use
limit daily dose to 2g
Non-narcotic analgesics
activate narcotic receptors in the brain or spinal cord
act on neurotransmitters, norepinephrine and serotonin
used first line
lower cost
many available OTC
for mild to moderate pain
Action: Inhibits prostaglandin production in the CNS
Roles in therapy: Treatment of headache, pain or fever, in combination with narcotics for moderate-to-severe pain
No noticeable anti-inflammatory effects
Side effects
Upset stomach
Vomiting
Contraindications
Severe liver impairment
Severe active liver disease
Cautions and considerations
Liver toxicity
Doses > 4 g per day
Chronic use at daily maximum doses
Permanent liver damage with intentional overdose
Alcohol use
Patients should limit daily dose of acetaminophen from 4 g to 2 g
Aspirin
Roles in Therapy
Mild-to-moderate pain and fever in patients >16 years old is associated with reays syndrome
Pain associated with inflammation
Stroke and heart attack prevention (low dose)
Used in combination with narcotics for moderate-to-sever pain
Actions: inhibits cyclooxygenase, the enzyme that converts arachidonic acid to prostaglandins
Side Effects
Upset stomach, GI Irritation and erosion, bleeding, headache, dizziness, skin rash, initiation or exacerbation of gout
Contraindications
Hypersensitivity to salicylates or other NSAIDS;
Asthma, rhinitis, nasal polyps; inherited or acquired bleeding disorders
Children <16 recovering from viral infections (potential Reye’s Syndrome)
Pregnancy
Cautions and Considerations
Not to be use in patients with bleeding disorders or a history of ulcers or an aspirin allergy
Drug interactions: other NSAIDS
80mg vs 81 mg
*ASA will smell like vinegar if it is outdated
What is a caution associated with acetaminophen use?
Do not take more than 4g per day
Do not use chronically at the daily maximum dose
Limit daily dose to 2g if alcohol is consumed regularly
18.2 Opioids and Pain Management
Opioid Analgesics
An analgesic is a drug that alleviates pain.
The opioid drug class includes morphine, codeine, and their semisynthetic or synthetic derivatives.
Opiate refers only to naturally occurring forms of the opioids.
The term narcotic is used in relation to health Canada
Opioid effects include analgesia, anxiety reduction sedation euphoria or dysphoria
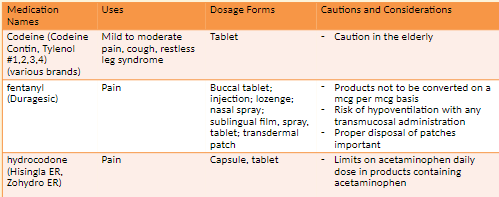
Narcotic Analgesics: codeine and morphine
Considered natural opiates and derived from the poppy plant
Codeine used to treat moderate pain
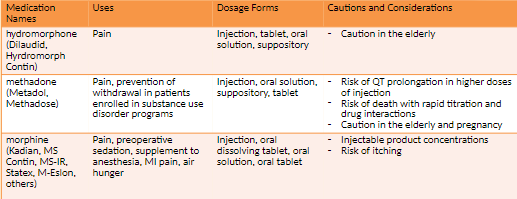
Morphine used to treat moderate-to-severe
Dosing and Administering Opioids
Morphine is the standard by which other opioids are measured.
An equianalgesic dose offers the same amount of analgesia.
MME is morphine milligram equivalent and can be found using conversion charts.
Opioids do not have a target dose and are titrated.
Routes include oral, rectal, intramuscular, subcutaneous, intravenous, patient-controlled analgesic (PCA) pump (IV), transdermal.
Potency: intravenous > intramuscular = subcutaneous > oral = rectal

Opioid Cautions and considerations
Caution in
Liver or kidney disease
Respiratory depression
Thyroid dysfunction, head injury, mental health disorders
Caution with use of other CNS depressants
Not to be used in substance disorder
Risk of:
Neonatal opioid withdrawal syndrome in exposed infants at birth
Hypogonadism
Drug interactions with drugs that slow opioid metabolism
Serotonin syndrome when combined with drugs affecting serotonin
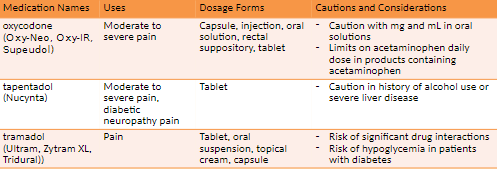
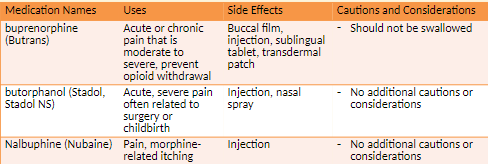
Combination Opioids
non-narcotic + narcotic = good alternative
less narcotic is needed
acts on 2 components of pain
How they work
Relieve pain centrally and peripherally
Purpose
Increase pain relief through drug synergy
Limit intake of addictive substances
Dose limits
Due to serious risk of aspirin or acetaminophen toxicity, daily limit is 4 g.
Content of acetaminophen now limited to 325 mg per tablet or capsule
Filling prescriptions for combination drugs
Strengths of both components must agree with prescription.
CII may not be refilled
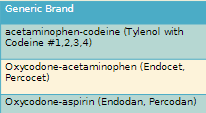
18.3 Opioid use Disorder and Treatments
Opioid Use Disorder and Treatments
Dependence: A physical and emotional reliance on a drug; Withdrawal when drug is discontinued or dose is reduced
keep using not as much concern
Addiction: Compulsive disorder leading to continued use of drug despite harm
Symptoms include preoccupation with drugs, refusal to taper off medication, strong preference for specific opioid, decreased ability to function.
Forged prescriptions, prescription loss, unsanctioned dose escalation, reports of shorted medication supply may indicate addiction
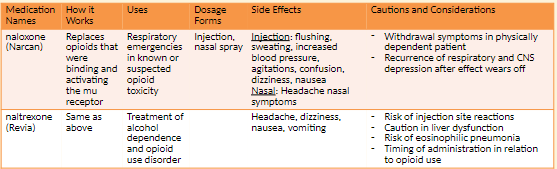
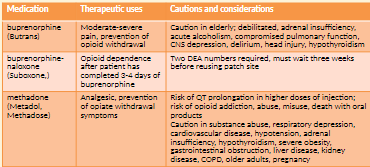
Opioid Antagonists (Naloxone) NBCP Directive
The provision of naloxone should be considered a necessary and appropriate part of the care plan for individuals experiencing/at risk of opioid overdose. This therapeutic option should be made available without regard to origin of the opioid (pursuant to prescription or via illicit means).
Any risk of medication side effects (e.g. immediate withdrawal, injection site infection, etc.) are outweighed by the benefit of prolonging life. Naloxone availability __has not__been shown to
increase risk taking behaviour, cannot be abused and causes no effect in absence of opioids.Barriers to appropriate availability in New Brunswick include:
Individuals with addiction experience stigma in society and healthcare which potentially increases risk of not receiving life-saving treatment
Lack of education within community regarding the availability of naloxone
Rural population may experience delay in first responder administration of naloxone
Practitioner failure to integrate the agent into individual client care plans
Naloxone acquisition cost
Naloxone plays a small but important role in the general strategy to address the major health crisis of opioid addiction. Pharmacy professionals in conjunction with other healthcare providers must act to ensure opioids are prescribed rationally according to established evidence so that life-threatening respiratory (and other major) sequelae are avoided.
TLDR anyone can purchase Naloxone with counseling from a pharmacist
In mid-2016, the National Drug Scheduling Advisory Committee (NDSAC) granted naloxone Schedule II (available without a prescription) status on the National Drug Schedules (NDS). At the end of December 2016 the nasal spray formulation of naloxone also gained Schedule II status.
Pharmacists and Pharmacy Technicians are encouraged to advocate for their clients in procurement of naloxone and work to surmount any barriers to access (including but not limited to economic, awareness, social, cultural and geographical).
Product Preparation: Regardless of administration route selected (intranasal or injection), pharmacy should provide a complete kit that contains written instructions (reinforcing in-person teaching) for administration and disposal, equipment (syringes, swabs, nasal atomiser etc.), along with the medication. Generally, multiple doses are provided in the event the initial dose effect wanes prior to the individual receiving emergent medical care.
Anesthetics
General anesthesia actions
Unconsciousness, analgesia (relieving pain), skeletal muscle relaxation, amnesia on recovery
General anesthesia assessment
Blood pressure, plasma volume, oxygen level, pulse, respiratory rate, tissue perfusion, urinary output
General anesthetics: preanesthetic medications
Narcotics alleviate pain and depress the respiratory center.
Benzodiazepines provide sedation, amnesia, and anti-anxiety effects
Allows painless and controlled surgical, obstetric, and diagnostic procedures
Most potent anesthetics are gases or vapors
Two classes of anesthesia: general and local
One anesthetic may be superior to another, depends on clinical situation
Final choice based on drugs and anesthetic techniques safest for patient
Physiologic Effects of Anesthesia
Involves many systems
Nervous
Respiratory
Endocrine
Cardiovascular
Skeletal muscular
GI
Hepatic
Goals of Balanced Anesthesia
Amnesia to eliminate patient’s memory of procedure
Adequate muscle relaxation, no contracting of muscles
Adequate ventilation by maintaining oxygen concentration
Pain control
Anesthesia: an intervention for intentional loss of feeling in a person’s body or part of the body when pain relief, anxiety relief, or muscle relaxation is required for patient care.
General anesthesia is the unique condition of reversible unconsciousness and absence of response to painful stimuli
General anesthesia: causes reversible unconsciousness and absence of response to painful stimuli.
Neuraxial anesthesia: blocks sensation through injection of an anesthetic into the CNS without making the patient unconscious.
ex. epidural
Local anesthesia: is used for local pain reduction
anesthesiologist oversees administration of anesthesia.
Reversible conditions of general anesthesia
Unconsciousness, analgesia, paralysis, amnesia
Assessing patient status during general anesthesia
Oxygenation, ventilation, circulation, temperature
Preanesthetic medications
Control sedation
Reduce postoperative pain
Provide amnesia
Decrease anxiety
Drugs often used: narcotics, benzodiazepines, phenothiazines
General anesthetics: malignant hyperthermia
A rare but serious side effect of anesthesia
A sudden and rapid rise in body temperature with irregularities in heart rhythm and breathing Fever of 110°F or more
Life-threatening and must be treated immediately
Treated with dantrolene (Dantrium), a skeletal muscle relaxant
Inhaled Anesthetics
General anesthetics: inhalation anesthetics
Stored in steel containers as compressed gas or liquid
Inhaled through a facemask
Respiratory system excretes 80-90% of dose
Side effects include ↓ blood pressure, ↑ fluid in the blood, ↓ renal function, nausea, and vomiting
Administered through a facemask
Respiratory system excretes 80%–90% of dose
Side effects include ↓ blood pressure, hypervolemia, ↓ renal function, nausea, and
vomiting.
Drugs
nitrous oxide (N2O)
Memory loss, mildly slowed breathing
Not be used in hypovolemia, shock, pneumothorax, cardiac disease
isoflurane (florane)
none
Not to be used with CO2 absorbents
Risk of dose-related hypotension, respiratory depression, ↑ cerebrospinal fluid pressure
Not recommended in coronary artery disease
sevoflurane (sevorane)
Nausea, vomiting, drowsiness, agitation, cough, shivering
Risk of QT prolongation
Risk of dose-related hypotension or respiratory depression
Risk of hepatic injury if previously exposed to halogenated hydrocarbon anesthetics
Risk of preoperative seizures
Injectable Anesthetics
Include the ultrashort-acting barbiturates and benzodiazepines
Distribution
Very lipid soluble
Initially distributes to the brain, liver, kidneys; later distributes to fat and muscle
Body distribution lowers concentrations.
Most administered by IV drip
drugs
etomidate (Tomvi)
Used to supplement a weak anesthetic, short outpatient procedures, sedation in rapid sequence intubation
Risk of cardiac depression in elderly particularly in those with hypertension, risk of drug toxicity in renal impairment
fentanyl
Preoperative medication, analgesic, open-heart surgery, supplement in balanced anesthesia
High risk of addiction, misuse, abuse; risk of increased plasma concentration when used with CYP3A4 inhibitors; risk of respiratory depression in older adults and debilitated patients
ketamine (Ketalar)
not necessarily used for general anesthesia
You feel the pain but do not remember it
Extended emergence period, rash, nausea, vomiting, increased urination, Produces dissociative amnesia
Not for patients with history of alcohol abuse
Risk of respiratory depression and apnea with rapid administration or overdose
Risk of increased blood pressure
Risk of cardiac decompensation
propofol (Diprivan)
Maintain anesthesia and sedation, treat agitation in the ICU
Risk of cardiorespiratory effects and anaphylactic reactions
Fentanyl analogs
sufentanil: 5-10 times more potent than fentanyl
remifentanil: Shortest-acting opioid, rapid offset even after prolonged infusions during surgery
Anesthesia Induction Agents
Benzodiazepines
diazepam (Valium)
lorazepam (Ativan)
midazolam
Aggressive or hyperactive behavior; sleep-related effects like performing certain activities without awareness
Patients to be monitored and informed of unexpected adverse reactions
Caution in respiratory depression
Roles in Therapy
Inhibits pain or the conscious perception of pain
General anesthetics
Provide reversible loss of consciousness and perception of pain
Local anesthetics
Block transmission of pain signal to the CNS from a specific anatomic site
Choice of anesthetic
Determined by clinical need and patient safety
Anesthesiologist
Oversees administration of anesthesia during surgery
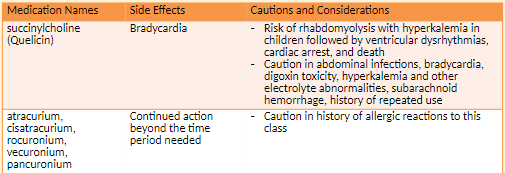
Neuromuscular Blocking Agents
Neuromuscular blocking agents paralyze skeletal muscles during surgery which enables a surgeon to operate with accuracy and safety.
These medications are also used as an adjunct to general anesthesia in endotracheal intubation
Dangerous due to immediate skeletal muscle paralysis
Processes for safe stocking and storage
Distinctly flag with an alert label during storage.
Do not store next to look-alike drugs
Causes immediate skeletal muscle relaxation of short, long, and extended durations
Stored in the refrigerator

General Anesthetics and Malignant Hyperthermia
Malignant hyperthermia is a sudden and rapid rise in body temperature with irregularities in heart rhythm and breathing.
It is a rare but serious side effect of anesthesia.
It may lead to cardiac arrest, brain damage, internal hemorrhage and requires immediate treatment
Malignant hyperthermia is treated with dantrolene (Dantrium, Revonto, Ryanodex), a skeletal muscle relaxant.
Hospitals are required to have a specialized drug kit immediately available for the treatment of malignant hyperthermia
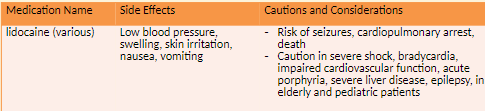
Local Anesthetics
Local anesthesia produces a transient and reversible loss of sensation in a defined area of the
body without altering alertness or mental function.Epinephrine is commonly used to keep the local anesthetic at the injection site.
Esters: are short-acting and are metabolized by pseudocholinesterase in the plasma.
Amides: are longer-acting and are metabolized by liver enzymes

Migraine
A neurological disorder commonly associated with an episodic and painful headache
Lasts a couple of hours to a couple of days
Headache is moderate to severe in intensity; pulses or throbs; can be accompanied by sensitivity to light, sound, and stimulation as well as vomiting and anorexia
Migraine pain is related to nociceptive pain pathways in the trigeminal ganglion
Description: A severe, throbbing, unilateral headache accompanied by symptoms such as nausea, vomiting, photophobia, phonophobia, hyperesthesia
caused by vasodilation of arteries in the brain
serotonin is one causative agent
caused by release of neuropeptides by the trigeminal nerve
Components of a migraine: Prodrome, aura, headache, headache relief, postdrome
Aura: Visual or sensory disturbances such as seeing flashing lights; shimmering heat waves; bright lights; dark halos in visual field; blurred, cloudy vision; transient loss of vision
Pathophysiology: Dilation of cerebral surface vessels
Increased levels followed by decreased levels of serotonin, a potent vasoconstrictor
Risk/ Causative factors: Diet, stress, sleep habits, certain medications, hormonal fluctuations, depression, atmospheric pressure changes, environmental irritants
Nondrug Therapy: Identifying and eliminating trigger factors, a quiet environment, sleep, lying down in a dark room
Prophylactic Therapy- maybe taken daily
antihypertensives
antidepressants
anticonvulsants
CGRP antagonist monoclonal antibodies
NSAIDs
SSRIs
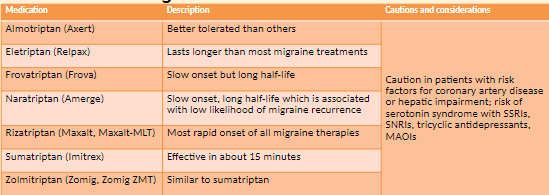
Acute or Abortive Therapy
simple analgesics
triptans
combinations of triptans with NSAIDs
antiemetics
lasmiditan
CGRP antagonists
ergots
Anti-Calcitonin gene-related peptide receptor (anti-CGRPR)
Medication-eren__umab__ (Aimovig)
NEW NOVEL BIOLOGIC
side effects- injection site reaction, constipation, muscle spasms and pruritus


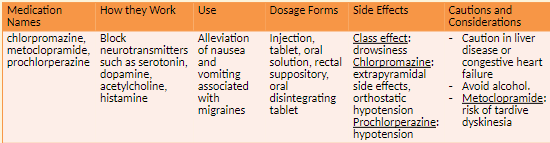
Antiemetic Agents
How it Works
Antiemetic Agents: Block neurotransmitters which cause nausea and vomiting
Examples of agents- metoclopramide
Side effects
drowsiness
Extrapyramidal effects and orthostatic hypotension with chlorpromazine
Cautions and Considerations
Caution with both in liver disease, congestive heart failure, concomitant use of alcohol; risk of tardive dyskinesia with metoclopramide
opioid analgesic- Butorphanol (Stadol) (Nasal Spray)
Other antimigraine agents: butalbital-aspirin-caffeine (Fiorinal, Fiorinal C1/4, C1/2)
Is a controlled Substance
Caution with both in pediatric patients or those with severe hepatic impairment
Complementary and Alternative Medicine
Caffeine
A CNS stimulant used in combination with other analgesics to treat headache
Also used to treat fatigue and drowsiness
Should not be used more than every three to four hours
Side effects: rapid heartbeat, palpitations, insomnia, restlessness, ringing in the ears, tremors, lightheadedness, nausea, vomiting, stomach pain, itchy skin rash
Capsaicin
Used as a topical treatment for pain
Some effectiveness in diabetic neuropathy, arthritis, and headache pain
When applied, burning, itching, and tingling occur followed by analgesia.
Gloves to be used during application and hands washed afterward
Feverfew
Used orally to prevent migraine pain
Improves nausea, vomiting, light sensitivity experienced during a migraine
Also used to treat menstrual cramps and arthritis
Side effects: heartburn, nausea, diarrhea, constipation, abdominal pain, gas
Cannabis sativa
marijuana
Synthetic and natural extracts used for chronic or neuropathic pain
Side effects: dizziness, weight gain, heart disease
Opioid Agonist Treatment
Opioid use disorder (OUD)
Principles: Patients receiving OAT services must be treated with respect and dignity
Pharmacists must employ the most recent OAT-related clinical practice guidelines, evidence and standards of practice to facilitate quality patient care.
Pharmacy practice must align with provincial and national harm reduction strategies
Safeguards are needed against diversion of agents used in OAT
Pharmacy professionals must comply with all applicable existing provincial and federal legislation, policy, and evidence-based clinical practice guidelines in addition to the NBCP Practice Directive.
The agents commonly used in the provision of OAT services are classified as narcotics and are subject to specific legislative requirements as set out in Schedule I of the Controlled Drugs and Substances Act.
Buprenorphine-Naloxone: This therapeutic agent has been shown to be lower risk to patients in terms of safety. The potential for diversion exists however overdose is less likely to be fatal.
Methadone: Evidence indicates methadone is associated with medication incidents (errors and near-miss events), as well as patient complaints to the College. These incidents and complaints indicate a significant degree of actual or potential harm from errors in the dispensing process and diversion. The pharmacologic and pharmacokinetic characteristics of methadone presents significant risk of overdose in opioid-naïve individuals.
Emerging treatment options
Sustained Release Oral Morphine (SROM): Due to the risks of toxicity associated with bolus absorption and diversion, extra safeguards need to be in place and are referred to in the NBCP directive.
iOAT: This treatment is not considered first line of therapy, however, injectable hydromorphone is a newer agent considered for use in OUD. It is considered to have high potential for diversion and risk to patient outcomes. More specific requirements of iOAT are included in the NBCP directive.
Deciding to offer OAT services:
OAT involves a high degree of patient interaction,
prescriber collaboration
preparation complexity,
clinical and dispensing documentation,
vigilance and commitment.
Roles of a pharmacy technician
Provide technical support as per, “Model Standards of Practice for Pharmacists and Pharmacy Technicians in Canada”
Process and prepare prescriptions for patients requiring OAT
Follow-up monitoring of patients for compliance with oral/buccal administration.
In institutional settings, observed dosing may be performed by professional staff as per institutional policy
Pharmacist
Provide patient care as per “Model Standards of Practice for Pharmacists and Pharmacy Technicians in Canada”
Establish a therapeutic pharmacist-patient relationship
Prescribe, sell, provide or transfer OAT as per Health Canada’s Section 56 Exemption 4
Follow-up monitoring of patients for compliance.
In institutional settings, observed dosing may be performed by professional staff as per institutional policy
Pharmacy manager
Manage the pharmacy as per, “Model Standards of Practice for Pharmacists and Pharmacy Technicians in Canada”, relevant provincial and federal legislation and this practice directive.
Establish pharmacy team commitment to providing care to patients with OUD
Complete and document preparations completed for implementation of OAT services at the pharmacy
Execute (or delegate management of) an ongoing quality management program (QMP) relating to OAT as a component of the pharmacy’s larger QMP.
When appropriate, inform and educate patients/community regarding OAT. Information and advertisement must conform to federal and provincial legislation.
Code of Ethics
Do not forget the CoE 4 main principles
Beneficence - to benefit
Non-Maleficence - do no harm, and prevent harm from occurring
Respect for Persons
Justice
Education and Training
Managers and other professional staff are encouraged to complete one of the courses outlined in the directive by NBCP.
All pharmacy professionals must also ensure competency in dealing with adverse patient outcomes associated with OAT.
Pharmacy practitioners must be competent in dealing with opioid overdose and the use of naloxone
Managers and other professional staff are encouraged to complete one of the courses outlined in the directive by NBCP.
All pharmacy professionals must also ensure competency in dealing with adverse patient outcomes associated with OAT.
Pharmacy practitioners must be competent in dealing with opioid overdose and the use of naloxone
Pharmacists who provide injectable hydromorphone (iOAT) services: The pharmacy manager (or their delegate) plus one other pharmacist in the practice (where more than one professional is employed in the practice) must complete one of the courses listed in Appendix B of the NBCP directive as a basis for engaging in providing iOAT.
Pharmacy managers are accountable for ensuring staff competency is documented.
Equipment and Storage
Equipment monitoring, calibration and maintenance must be included in the pharmacy’s quality management program (QMP).
Measuring devices for preparation of methadone must be calibrated such that the error rate is no greater than 0.1mL therefore, the use of graduated cylinders is not acceptable for measuring of methadone. Typically, oral syringes or manual/electronic pumps that are regularly calibrated as per manufacturer specification meet this accuracy requirement.
Equipment used to prepare methadone may not be used to prepare or compound other medications. ** must be identified as dedicated only to methadone preparation.
All medications used in provision of OAT must not be visible from outside the dispensary and must be stored securely until provided to the patient
Patient Access to Pharmacy
Patients requiring daily observed dosing must access a pharmacist every day.
Where seven day per week service is not possible, pharmacy managers must accommodate patients who are not assessed as appropriate for receiving take-home 6 doses.
Options include:
Allowing patients access during short window of time to allow for daily assessment and administration
Coordinating “closed day” observed dosing with a secondary pharmacy.
Emergency planning
Documentation
Pharmacy managers are responsible for the development or adaptation of forms to document clinical activities, steps completed in the dispensing process and for setting expectations of the patient-pharmacy professional relationship.
Dispensing documentation: Pharmacists and pharmacy technicians must document each step in dispensing of OAT. Given that medication used in OUD may require dilution, and that the agents are desirable for purposes of diversion, the documentation required is greater than that required for dispensing of typical (non-OAT) oral dosage forms
Clinical documentation: Pharmacists must document patient assessment. Pharmacists or pharmacy technicians must document follow-up of patients for observed dosing or take-home dispensing
College documentation: Provision of OAT does not require authorization from the College however, the College must be notified of provision of OAT service by the pharmacy manager via online declaration within the
pharmacy’s profile. This information may be made publicly available to assist patients in finding care
Quality Management Program (QMP)
Pharmacies are required to have a QMP in place according to the regulations and the Model Standards of Practice for Pharmacists and Pharmacy Technicians in Canada
QMP documentation relating to OAT is a required component of the pharmacy’s overall QMP records.
Pharmacy managers are responsible (in collaboration with staff) for establishing/ adapting forms to meet the needs of their unique practice as well as updating, monitoring and retaining these forms
Prescription Standards and Requirements
who must prescribe OAT
Federal law and provincial policy establish prescribers of controlled substances, and are defined in:
the Controlled Drug and Substance Act(CDSA)
New Classes of Practitioners Regulations
as a class exemption for patients, practitioners and pharmacists prescribing and providing controlled substances in Canada
ALL prescription Requirements set out in federal and provincial laws, regulations and policies for drugs listed on Schedule I of the CDSA apply to therapeutic agents used in OAT.
Methadone, SROM and iOAT: MUST include start and stop dates and indicate observed dosing.
Methadone: Prescriptions for methadone must include details regarding take-home dosing schedules.
In standards, act is all listed when the prescription is given the physician will write exact start and stop dates and indicates “carries” take hom
Computer Processing
Observed doses entered into computer software and the patient’s electronic health record (EHR), but subsequently not administered to patients, must be reversed before the pharmacy closes for the day to ensure accuracy of the patient’s EHR.
This provides accurate data to other health care providers who may be caring for the patient. The prescription actual stop date (mandatory for methadone, SROM and iOAT) as expressed by the prescriber must be entered into the software. The software may require that the stop date be entered manually.
the carries must be billed on a daily basis with the stop dates
Labeling of Observing Dosing
Observed doses are exempt from labeling if prepared and immediately provided to the patient, where no opportunity for dosing or patient identification error exists.
OAT prepared ahead of time for dispensing as observed doses must be labelled, and that label must contain, at a minimum:
the name of the drug
the dose
lot number
expiry date from the stock bottle/original packaging
pharmacy team member who prepared the dose.
All labels on take-home doses must:
Comply with legal requirements
Include number of remaining part-fills, and the prescriber's stop date
Methadone
labels for take-home doses must:
Conform to practice guidelines CAMH: Pharmacist’s Guide
Include beyond-use-date (BUD) as determined by existing stability data.
Specify on the first line of the signature (sig) line “Drink the full contents of bottle (XX mg) once daily”. This ensures the dispensing record created within the patient’s EHR explicitly communicates the dose prescribed to any member of the patient care team.
Preparation of Final Dose: Buprenorphine/Naloxone
Preparation of observed and take-home dose packaging must maintain integrity of the dosage form.
Methadone Final Dose
Must be single-use daily dose regardless of patient care setting.
Calculations must be performed by a pharmacist or pharmacy technician.
Commercially available 10 mg/ml oral stock solutions must be used (a consistent manufacturer for the stock methadone solution is encouraged as dispensing of the same brand may allow patients to identify change in taste, which may signal a potential dosage error, changes in manufacturer of stock solution must be communicated to patients)
Stock solution must be diluted to total volume of approximately 100 ml with a vehicle such as Tang®, Allen’s apple juice or another crystalline juice.
Diluent juice must be refrigerated and labeled with name, date and before use date of preparation.
Must be dispensed in a light-resistant and child-resistant packaging.
Must be dispensed in a tamper-evident bottle. This facilitates take home audits and identification of misuse.
SROM Final Dose
Use SROM with 24-hour dose interval (not a product intended for 12-hour dosing)
The capsule must be opened to release the enclosed pellets for immediate consumption.
The pellets must be swallowed whole. Crushing, chewing, or dissolving slow-release oral morphine capsules or pellets can cause rapid release and absorption of a potentially fatal dose of morphine
The pellets are to be sprinkled onto a small amount of applesauce or pudding. Alternatively, pellets may be transferred into a medicine cup and ingested, followed by water to ensure all pellets have been swallowed
*is very specific
iOAT Final Dose
A pharmacist must perform the follow-up monitoring after a patient self-administers iOAT.
This function may not be performed by a pharmacy technician or unregulated member of staff.
Take-Home Doses
If the prescriber has indicated take-home doses and the pharmacist, after patient assessment, believes there is a risk to the patient of overdose, misuse or diversion, the pharmacist may refuse to dispense take-home doses, and revert to observed dosing. .
This intervention must be documented, and the prescriber must be informed.
Buprenorphine/naloxon
The maximum number of take-home doses is six.
Some patients may be sufficiently stable to allow for up to 13 take-home doses.
Take-home doses of buprenorphine-naloxone are not required to be provided in a “lock box”.
Methadone
The “lock box” should be discreet so as not to draw attention to the patient.
cash box with a key
used as child proofing
The lock box must be made of materials which will impede access to the methadone and decrease the risk of unintended ingestion by children and opioid-naïve individuals.
Softsided containers, which can be cut with a blade or scissors, are not appropriate.
Each patient must have their own identifiable “lock-box”
Patients must be informed that they may be asked at any time to appear in the pharmacy and bring with them the remainder of their take-home doses and empty bottles, in their own lock box.
This procedure, referred to as a "carry audit", may be used to assess for diversion or misuse
Take-Home Doses
SROM: To limit potential for diversion, slow-release oral morphine is only to be provided via daily observed ingestion
iOAT: To limit potential for diversion and encourage safe injection technique, iOAT is only to be provided via daily observed self-administration in accordance with policy and practice guidelines
Follow-up Adherence Monitoring Methadone
Methadone packaging may be used for illicit purposes.
To prevent diversion of methadone packaging in community pharmacy, take-home bottles must be returned to the pharmacy prior to new take-home doses being issued.
A pharmacy team member must reconcile the empty bottles returned with the number that were issued and this reconciliation must be documented.
In institutional settings, any packaging of methadone should be discarded according to institutional policy.
document that a bottle is missing and do not give them more for home tell the physician
Some patients may occasionally experience extenuating circumstances such as short-term illness or life events (e.g. public health emergencies, travel, bereavement or temporary employment demands) that present a barrier to attending a pharmacy for patient care and supply of OAT. Pharmacy professionals faced with challenging decisions regarding how to balance patient benefit with public safety should apply the Values Based Decision Model (VBDM) as described within the College’s Code of Ethics. Decision rationale must be documented within the patient profile to facilitate Intraprofessional care.
Extenuating Patient Circumstances – Potential Scenarios
Delivery:
If a patient is unable to attend the pharmacy due to extenuating circumstances OAT may be delivered, as per Health Canada.
The pharmacist must ensure the patient is assessed prior to the dose administration and follow-up monitoring subsequently occurs
Documentarian of OAT
Dispensing Documentation
Pharmacists or pharmacy technicians must record and retain documentation of the steps in preparation and provision of OAT as per standards of practice and regulations.
Pharmacy professionals (community pharmacy only) must document the return of take-home methadone bottles.
Clinical Documentation
Pharmacists must record and retain documentation of their patient assessment, clinical decision-making (including documentation of care decisions made in response to exceptional circumstances)
and follow-up monitoring as per established standards of practice and regulation
anything done and is beyond pharmacy documentation has to be done and possible sent to the physician in the computer system in the patients file
Inter/Intraprofessional Documentation
Prescription Monitoring Program (PMP) Through electronic health record (EHR)
The dose and the date of last observed dose must be documented in the PMP, if a dose is not administered as expected, before end of the day, the pharmacy must ensure the dispensing record is amended to ensure accuracy (use alternate means of communication (telephone, secure electronic messaging or in-person discussion) to ensure safe patient care.
Inter/Intraprofessional documentation of the provision of OAT is vital to the safe and effective care of patients as they transition between care environments and providers.
Transition of Custody
Pharmacy professionals may collaborate with other health care professionals to provide patient-specific prepared and labeled doses of OAT to an externally located patient care site. This is considered a transfer of custody.
The following professionals can be involved in administering OAT to patients and may possess patient doses of OAT:
Another pharmacist
Hospital employee authorized to receive substances listed within Schedule 1 of the CDSA
Medical practitioner (physician)
Nurses practicing as per Health Canada’s Subsection 56(1) (Class Exemption for Nurses Providing Health Care at a Community Health Facility)
Nurse practitioners as per (Section 2 of the New Classes of Practitioners Regulations to Controlled Drugs and Substances Act)
Pharmacy professionals must facilitate patient and community safety as best possible by supporting these providers to:
Confirm receipt of doses through a signature of the health care professional
Accomplish dose adjustments through provision of a new pharmacy- prepared dose
Avoid alteration of patient-labeled doses
Avoid altering dose volumes for administration
Prohibit use of a dose intended for one patient by another
Return unused patient doses within 72 hours
OAT Quality Management Program (QMP)
Education and Training Documentation: Pharmacy professionals must document within the pharmacy’s QMP that they have established and maintained competency for providing OAT for patients with OUD
Medication Incidents (Errors and Near-Misses) and Continuous Quality Improvement (CQI)
All medication incidents must be addressed according to the College’s “Practice Directive: Mandatory
Medication Incident Reporting (MMIR)” , the NAPRA Model Standards of Practice for Pharmacists and Pharmacy Technicians in Canada.
**Please refer to CAMH: Pharmacist’s Guide for specific management of OAT medication incident
OAT Quality Management Program
Equipment Provisions: ongoing monitoring of equipment used in providing OAT are outlined in the College’s Expectations for a Quality Management Program
Facilities Provisions: ongoing monitoring of the physical facilities where OAT is provided are outlined in the College’s Expectations for a Quality Management Program
Pharmacology test #3
18 Pain, Anesthesia and Drug Treatment
Physiology of Pain
Peripheral Nervous System: detects temperature, pressure, touch, pain
Sensation: Heat, cold, pressure, or chemical stimulus to sensory receptors and nerve endings in the peripheral nervous system
Pain Receptors: Perceive the sensation of pain when stimulated
Pain: as physically or emotionally sensed discomfort that is associated with acute tissue damage or a sensory system malfunction
Pain receptors, known as nociceptors, are activated when a stimulus could damage tissue.
Pain warns of damage or the presence of disease.
Pain is a normal part of healing
Pain Management
Pain
a normal physiologic response to stimuli usually associated with tissue damage
alerts the body to injury or inflammation
Inflammation: causes a cascade of events that untimely forms prostaglandins
Prostaglandins
chemical triggers for pain
causes local redness and swelling

Types of Pain
Somatic Pain: originates from injury to body tissue
ex. include post-surgical incision pain and bone pain from cancer metastases
Throbbing, stabbing, well localized
Narcotics, NSAIDs, nerve blockers
Visceral Pain: originates from problems with internal organs
Signals not received from these organs by sensory nerves
Symptoms include nausea, vomiting, and sweating, Aching, throbbing, sharp, gnawing, crampy, deep squeezing; associated with sweating, nausea, vomiting
Narcotics, NSAIDs, nerve blockers, antiemetics
Neuropathic Pain: from damage of nerve tissue
Symptoms include a tingling, burning, or stabbing pain in area of injury; pain frequently radiates.
Burning, aching, numbing, tingling, viselike, knifelike, constant
Antidepressants, anticonvulsants
Sympathetic-mediated pain; centralized: Patient feels pain when there is no obvious stimulus
Example is phantom-limb pain in which pain is felt in a limb that is no longer there.
Occurring when no pain should be felt
Nerve blockers
Timing of Pain
Acute Pain
Associated with trauma or surgery
Occurs within 6 weeks of a pain-inducing event
Has a beginning and an end, warns of a problem
Pain that lasts less than six months
Ranges from mild to severe
Sympathetic nervous system response includes increased pulse, blood pressure, and breathing rate; tensing of muscles; dilation of pupils
Chronic nonmalignant Pain
Cause may or may not be diagnosed.
Lasts > 3-6 months, may respond poorly to treatment
Does not cease when an illness or injury is healed
Compensatory response includes feelings of hopelessness, lack of facial expression, isolation, fatigue, anger, physical inactivity.
Chronic Malignant Pain
accompanies malignant disease
Increases in severity as diseases progresses
Response to Pain
Background pain: describes a constant level of pain.
Breakthrough pain:describes when pain of greater intensity occurs intermittently for no particular reason.
Provoked pain: describes pain that is more intense than background pain but has a clear cause.
Pain Assessment Scales




Categories of Drugs for Pain:
Narcotics- severe pain
non-narcotics- moderate/mind pain
NSAIDs- moderate/mild pain
The “Ideal” Analgesic
would…
provide maximum pain relief
produce no side effects
cause no dependence or addiction
Mild-to-moderate Pain Disorders
mild pain: described as 1 to 4 on a numeric rating scale
moderate pain: a 5-6 on a numeric rating scale
causes of mild-to-moderate pain: a variety of disorders including headaches
Treatment: non-narcotic Angelic
work independently of opioid receptors
mild-to-moderate trying to treat without narcotics
Non-narcotic Analgesics: Acetaminophen
liver toxicity
Doses > 4 g per day
Chronic use at daily maximum doses
Permanent liver damage with intentional overdose
Alcohol Use
limit daily dose to 2g
Non-narcotic analgesics
activate narcotic receptors in the brain or spinal cord
act on neurotransmitters, norepinephrine and serotonin
used first line
lower cost
many available OTC
for mild to moderate pain
Action: Inhibits prostaglandin production in the CNS
Roles in therapy: Treatment of headache, pain or fever, in combination with narcotics for moderate-to-severe pain
No noticeable anti-inflammatory effects
Side effects
Upset stomach
Vomiting
Contraindications
Severe liver impairment
Severe active liver disease
Cautions and considerations
Liver toxicity
Doses > 4 g per day
Chronic use at daily maximum doses
Permanent liver damage with intentional overdose
Alcohol use
Patients should limit daily dose of acetaminophen from 4 g to 2 g
Aspirin
Roles in Therapy
Mild-to-moderate pain and fever in patients >16 years old is associated with reays syndrome
Pain associated with inflammation
Stroke and heart attack prevention (low dose)
Used in combination with narcotics for moderate-to-sever pain
Actions: inhibits cyclooxygenase, the enzyme that converts arachidonic acid to prostaglandins
Side Effects
Upset stomach, GI Irritation and erosion, bleeding, headache, dizziness, skin rash, initiation or exacerbation of gout
Contraindications
Hypersensitivity to salicylates or other NSAIDS;
Asthma, rhinitis, nasal polyps; inherited or acquired bleeding disorders
Children <16 recovering from viral infections (potential Reye’s Syndrome)
Pregnancy
Cautions and Considerations
Not to be use in patients with bleeding disorders or a history of ulcers or an aspirin allergy
Drug interactions: other NSAIDS
80mg vs 81 mg
*ASA will smell like vinegar if it is outdated

What is a caution associated with acetaminophen use?
Do not take more than 4g per day
Do not use chronically at the daily maximum dose
Limit daily dose to 2g if alcohol is consumed regularly
18.2 Opioids and Pain Management
Opioid Analgesics
An analgesic is a drug that alleviates pain.
The opioid drug class includes morphine, codeine, and their semisynthetic or synthetic derivatives.
Opiate refers only to naturally occurring forms of the opioids.
The term narcotic is used in relation to health Canada
Opioid effects include analgesia, anxiety reduction sedation euphoria or dysphoria
Narcotic Analgesics: codeine and morphine
Considered natural opiates and derived from the poppy plant
Codeine used to treat moderate pain
Morphine used to treat moderate-to-severe
Dosing and Administering Opioids
Morphine is the standard by which other opioids are measured.
An equianalgesic dose offers the same amount of analgesia.
MME is morphine milligram equivalent and can be found using conversion charts.
Opioids do not have a target dose and are titrated.
Routes include oral, rectal, intramuscular, subcutaneous, intravenous, patient-controlled analgesic (PCA) pump (IV), transdermal.
Potency: intravenous > intramuscular = subcutaneous > oral = rectal

Opioid Cautions and considerations
Caution in
Liver or kidney disease
Respiratory depression
Thyroid dysfunction, head injury, mental health disorders
Caution with use of other CNS depressants
Not to be used in substance disorder
Risk of:
Neonatal opioid withdrawal syndrome in exposed infants at birth
Hypogonadism
Drug interactions with drugs that slow opioid metabolism
Serotonin syndrome when combined with drugs affecting serotonin




Combination Opioids
non-narcotic + narcotic = good alternative
less narcotic is needed
acts on 2 components of pain
How they work
Relieve pain centrally and peripherally
Purpose
Increase pain relief through drug synergy
Limit intake of addictive substances
Dose limits
Due to serious risk of aspirin or acetaminophen toxicity, daily limit is 4 g.
Content of acetaminophen now limited to 325 mg per tablet or capsule
Filling prescriptions for combination drugs
Strengths of both components must agree with prescription.
CII may not be refilled

18.3 Opioid use Disorder and Treatments
Opioid Use Disorder and Treatments
Dependence: A physical and emotional reliance on a drug; Withdrawal when drug is discontinued or dose is reduced
keep using not as much concern
Addiction: Compulsive disorder leading to continued use of drug despite harm
Symptoms include preoccupation with drugs, refusal to taper off medication, strong preference for specific opioid, decreased ability to function.
Forged prescriptions, prescription loss, unsanctioned dose escalation, reports of shorted medication supply may indicate addiction


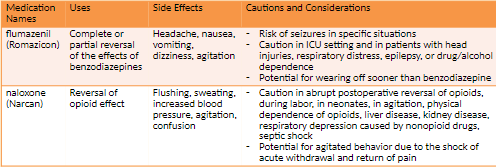
Opioid Antagonists (Naloxone) NBCP Directive
The provision of naloxone should be considered a necessary and appropriate part of the care plan for individuals experiencing/at risk of opioid overdose. This therapeutic option should be made available without regard to origin of the opioid (pursuant to prescription or via illicit means).
Any risk of medication side effects (e.g. immediate withdrawal, injection site infection, etc.) are outweighed by the benefit of prolonging life. Naloxone availability __has not__been shown to
increase risk taking behaviour, cannot be abused and causes no effect in absence of opioids.Barriers to appropriate availability in New Brunswick include:
Individuals with addiction experience stigma in society and healthcare which potentially increases risk of not receiving life-saving treatment
Lack of education within community regarding the availability of naloxone
Rural population may experience delay in first responder administration of naloxone
Practitioner failure to integrate the agent into individual client care plans
Naloxone acquisition cost
Naloxone plays a small but important role in the general strategy to address the major health crisis of opioid addiction. Pharmacy professionals in conjunction with other healthcare providers must act to ensure opioids are prescribed rationally according to established evidence so that life-threatening respiratory (and other major) sequelae are avoided.
TLDR anyone can purchase Naloxone with counseling from a pharmacist
In mid-2016, the National Drug Scheduling Advisory Committee (NDSAC) granted naloxone Schedule II (available without a prescription) status on the National Drug Schedules (NDS). At the end of December 2016 the nasal spray formulation of naloxone also gained Schedule II status.
Pharmacists and Pharmacy Technicians are encouraged to advocate for their clients in procurement of naloxone and work to surmount any barriers to access (including but not limited to economic, awareness, social, cultural and geographical).
Product Preparation: Regardless of administration route selected (intranasal or injection), pharmacy should provide a complete kit that contains written instructions (reinforcing in-person teaching) for administration and disposal, equipment (syringes, swabs, nasal atomiser etc.), along with the medication. Generally, multiple doses are provided in the event the initial dose effect wanes prior to the individual receiving emergent medical care.
Anesthetics
General anesthesia actions
Unconsciousness, analgesia (relieving pain), skeletal muscle relaxation, amnesia on recovery
General anesthesia assessment
Blood pressure, plasma volume, oxygen level, pulse, respiratory rate, tissue perfusion, urinary output
General anesthetics: preanesthetic medications
Narcotics alleviate pain and depress the respiratory center.
Benzodiazepines provide sedation, amnesia, and anti-anxiety effects
Allows painless and controlled surgical, obstetric, and diagnostic procedures
Most potent anesthetics are gases or vapors
Two classes of anesthesia: general and local
One anesthetic may be superior to another, depends on clinical situation
Final choice based on drugs and anesthetic techniques safest for patient
Physiologic Effects of Anesthesia
Involves many systems
Nervous
Respiratory
Endocrine
Cardiovascular
Skeletal muscular
GI
Hepatic
Goals of Balanced Anesthesia
Amnesia to eliminate patient’s memory of procedure
Adequate muscle relaxation, no contracting of muscles
Adequate ventilation by maintaining oxygen concentration
Pain control
Anesthesia: an intervention for intentional loss of feeling in a person’s body or part of the body when pain relief, anxiety relief, or muscle relaxation is required for patient care.
General anesthesia is the unique condition of reversible unconsciousness and absence of response to painful stimuli
General anesthesia: causes reversible unconsciousness and absence of response to painful stimuli.
Neuraxial anesthesia: blocks sensation through injection of an anesthetic into the CNS without making the patient unconscious.
ex. epidural
Local anesthesia: is used for local pain reduction
anesthesiologist oversees administration of anesthesia.
Reversible conditions of general anesthesia
Unconsciousness, analgesia, paralysis, amnesia
Assessing patient status during general anesthesia
Oxygenation, ventilation, circulation, temperature
Preanesthetic medications
Control sedation
Reduce postoperative pain
Provide amnesia
Decrease anxiety
Drugs often used: narcotics, benzodiazepines, phenothiazines
General anesthetics: malignant hyperthermia
A rare but serious side effect of anesthesia
A sudden and rapid rise in body temperature with irregularities in heart rhythm and breathing Fever of 110°F or more
Life-threatening and must be treated immediately
Treated with dantrolene (Dantrium), a skeletal muscle relaxant
Inhaled Anesthetics
General anesthetics: inhalation anesthetics
Stored in steel containers as compressed gas or liquid
Inhaled through a facemask
Respiratory system excretes 80-90% of dose
Side effects include ↓ blood pressure, ↑ fluid in the blood, ↓ renal function, nausea, and vomiting
Administered through a facemask
Respiratory system excretes 80%–90% of dose
Side effects include ↓ blood pressure, hypervolemia, ↓ renal function, nausea, and
vomiting.
Drugs
nitrous oxide (N2O)
Memory loss, mildly slowed breathing
Not be used in hypovolemia, shock, pneumothorax, cardiac disease
isoflurane (florane)
none
Not to be used with CO2 absorbents
Risk of dose-related hypotension, respiratory depression, ↑ cerebrospinal fluid pressure
Not recommended in coronary artery disease
sevoflurane (sevorane)
Nausea, vomiting, drowsiness, agitation, cough, shivering
Risk of QT prolongation
Risk of dose-related hypotension or respiratory depression
Risk of hepatic injury if previously exposed to halogenated hydrocarbon anesthetics
Risk of preoperative seizures
Injectable Anesthetics
Include the ultrashort-acting barbiturates and benzodiazepines
Distribution
Very lipid soluble
Initially distributes to the brain, liver, kidneys; later distributes to fat and muscle
Body distribution lowers concentrations.
Most administered by IV drip
drugs
etomidate (Tomvi)
Used to supplement a weak anesthetic, short outpatient procedures, sedation in rapid sequence intubation
Risk of cardiac depression in elderly particularly in those with hypertension, risk of drug toxicity in renal impairment
fentanyl
Preoperative medication, analgesic, open-heart surgery, supplement in balanced anesthesia
High risk of addiction, misuse, abuse; risk of increased plasma concentration when used with CYP3A4 inhibitors; risk of respiratory depression in older adults and debilitated patients
ketamine (Ketalar)
not necessarily used for general anesthesia
You feel the pain but do not remember it
Extended emergence period, rash, nausea, vomiting, increased urination, Produces dissociative amnesia
Not for patients with history of alcohol abuse
Risk of respiratory depression and apnea with rapid administration or overdose
Risk of increased blood pressure
Risk of cardiac decompensation
propofol (Diprivan)
Maintain anesthesia and sedation, treat agitation in the ICU
Risk of cardiorespiratory effects and anaphylactic reactions
Fentanyl analogs
sufentanil: 5-10 times more potent than fentanyl
remifentanil: Shortest-acting opioid, rapid offset even after prolonged infusions during surgery
Anesthesia Induction Agents
Benzodiazepines
diazepam (Valium)
lorazepam (Ativan)
midazolam
Aggressive or hyperactive behavior; sleep-related effects like performing certain activities without awareness
Patients to be monitored and informed of unexpected adverse reactions
Caution in respiratory depression
Roles in Therapy
Inhibits pain or the conscious perception of pain
General anesthetics
Provide reversible loss of consciousness and perception of pain
Local anesthetics
Block transmission of pain signal to the CNS from a specific anatomic site
Choice of anesthetic
Determined by clinical need and patient safety
Anesthesiologist
Oversees administration of anesthesia during surgery
Neuromuscular Blocking Agents
Neuromuscular blocking agents paralyze skeletal muscles during surgery which enables a surgeon to operate with accuracy and safety.
These medications are also used as an adjunct to general anesthesia in endotracheal intubation
Dangerous due to immediate skeletal muscle paralysis
Processes for safe stocking and storage
Distinctly flag with an alert label during storage.
Do not store next to look-alike drugs
Causes immediate skeletal muscle relaxation of short, long, and extended durations
Stored in the refrigerator



General Anesthetics and Malignant Hyperthermia
Malignant hyperthermia is a sudden and rapid rise in body temperature with irregularities in heart rhythm and breathing.
It is a rare but serious side effect of anesthesia.
It may lead to cardiac arrest, brain damage, internal hemorrhage and requires immediate treatment
Malignant hyperthermia is treated with dantrolene (Dantrium, Revonto, Ryanodex), a skeletal muscle relaxant.
Hospitals are required to have a specialized drug kit immediately available for the treatment of malignant hyperthermia
Local Anesthetics
Local anesthesia produces a transient and reversible loss of sensation in a defined area of the
body without altering alertness or mental function.Epinephrine is commonly used to keep the local anesthetic at the injection site.
Esters: are short-acting and are metabolized by pseudocholinesterase in the plasma.
Amides: are longer-acting and are metabolized by liver enzymes


Migraine
A neurological disorder commonly associated with an episodic and painful headache
Lasts a couple of hours to a couple of days
Headache is moderate to severe in intensity; pulses or throbs; can be accompanied by sensitivity to light, sound, and stimulation as well as vomiting and anorexia
Migraine pain is related to nociceptive pain pathways in the trigeminal ganglion
Description: A severe, throbbing, unilateral headache accompanied by symptoms such as nausea, vomiting, photophobia, phonophobia, hyperesthesia
caused by vasodilation of arteries in the brain
serotonin is one causative agent
caused by release of neuropeptides by the trigeminal nerve
Components of a migraine: Prodrome, aura, headache, headache relief, postdrome
Aura: Visual or sensory disturbances such as seeing flashing lights; shimmering heat waves; bright lights; dark halos in visual field; blurred, cloudy vision; transient loss of vision
Pathophysiology: Dilation of cerebral surface vessels
Increased levels followed by decreased levels of serotonin, a potent vasoconstrictor
Risk/ Causative factors: Diet, stress, sleep habits, certain medications, hormonal fluctuations, depression, atmospheric pressure changes, environmental irritants
Nondrug Therapy: Identifying and eliminating trigger factors, a quiet environment, sleep, lying down in a dark room
Prophylactic Therapy- maybe taken daily
antihypertensives
antidepressants
anticonvulsants
CGRP antagonist monoclonal antibodies
NSAIDs
SSRIs
Acute or Abortive Therapy
simple analgesics
triptans
combinations of triptans with NSAIDs
antiemetics
lasmiditan
CGRP antagonists
ergots

Anti-Calcitonin gene-related peptide receptor (anti-CGRPR)
Medication-eren__umab__ (Aimovig)
NEW NOVEL BIOLOGIC
side effects- injection site reaction, constipation, muscle spasms and pruritus


Antiemetic Agents
How it Works
Antiemetic Agents: Block neurotransmitters which cause nausea and vomiting
Examples of agents- metoclopramide
Side effects
drowsiness
Extrapyramidal effects and orthostatic hypotension with chlorpromazine
Cautions and Considerations
Caution with both in liver disease, congestive heart failure, concomitant use of alcohol; risk of tardive dyskinesia with metoclopramide

opioid analgesic- Butorphanol (Stadol) (Nasal Spray)
Other antimigraine agents: butalbital-aspirin-caffeine (Fiorinal, Fiorinal C1/4, C1/2)
Is a controlled Substance
Caution with both in pediatric patients or those with severe hepatic impairment
Complementary and Alternative Medicine
Caffeine
A CNS stimulant used in combination with other analgesics to treat headache
Also used to treat fatigue and drowsiness
Should not be used more than every three to four hours
Side effects: rapid heartbeat, palpitations, insomnia, restlessness, ringing in the ears, tremors, lightheadedness, nausea, vomiting, stomach pain, itchy skin rash
Capsaicin
Used as a topical treatment for pain
Some effectiveness in diabetic neuropathy, arthritis, and headache pain
When applied, burning, itching, and tingling occur followed by analgesia.
Gloves to be used during application and hands washed afterward
Feverfew
Used orally to prevent migraine pain
Improves nausea, vomiting, light sensitivity experienced during a migraine
Also used to treat menstrual cramps and arthritis
Side effects: heartburn, nausea, diarrhea, constipation, abdominal pain, gas
Cannabis sativa
marijuana
Synthetic and natural extracts used for chronic or neuropathic pain
Side effects: dizziness, weight gain, heart disease
Opioid Agonist Treatment
Opioid use disorder (OUD)
Principles: Patients receiving OAT services must be treated with respect and dignity
Pharmacists must employ the most recent OAT-related clinical practice guidelines, evidence and standards of practice to facilitate quality patient care.
Pharmacy practice must align with provincial and national harm reduction strategies
Safeguards are needed against diversion of agents used in OAT
Pharmacy professionals must comply with all applicable existing provincial and federal legislation, policy, and evidence-based clinical practice guidelines in addition to the NBCP Practice Directive.
The agents commonly used in the provision of OAT services are classified as narcotics and are subject to specific legislative requirements as set out in Schedule I of the Controlled Drugs and Substances Act.
Buprenorphine-Naloxone: This therapeutic agent has been shown to be lower risk to patients in terms of safety. The potential for diversion exists however overdose is less likely to be fatal.
Methadone: Evidence indicates methadone is associated with medication incidents (errors and near-miss events), as well as patient complaints to the College. These incidents and complaints indicate a significant degree of actual or potential harm from errors in the dispensing process and diversion. The pharmacologic and pharmacokinetic characteristics of methadone presents significant risk of overdose in opioid-naïve individuals.
Emerging treatment options
Sustained Release Oral Morphine (SROM): Due to the risks of toxicity associated with bolus absorption and diversion, extra safeguards need to be in place and are referred to in the NBCP directive.
iOAT: This treatment is not considered first line of therapy, however, injectable hydromorphone is a newer agent considered for use in OUD. It is considered to have high potential for diversion and risk to patient outcomes. More specific requirements of iOAT are included in the NBCP directive.
Deciding to offer OAT services:
OAT involves a high degree of patient interaction,
prescriber collaboration
preparation complexity,
clinical and dispensing documentation,
vigilance and commitment.
Roles of a pharmacy technician
Provide technical support as per, “Model Standards of Practice for Pharmacists and Pharmacy Technicians in Canada”
Process and prepare prescriptions for patients requiring OAT
Follow-up monitoring of patients for compliance with oral/buccal administration.
In institutional settings, observed dosing may be performed by professional staff as per institutional policy
Pharmacist
Provide patient care as per “Model Standards of Practice for Pharmacists and Pharmacy Technicians in Canada”
Establish a therapeutic pharmacist-patient relationship
Prescribe, sell, provide or transfer OAT as per Health Canada’s Section 56 Exemption 4
Follow-up monitoring of patients for compliance.
In institutional settings, observed dosing may be performed by professional staff as per institutional policy
Pharmacy manager
Manage the pharmacy as per, “Model Standards of Practice for Pharmacists and Pharmacy Technicians in Canada”, relevant provincial and federal legislation and this practice directive.
Establish pharmacy team commitment to providing care to patients with OUD
Complete and document preparations completed for implementation of OAT services at the pharmacy
Execute (or delegate management of) an ongoing quality management program (QMP) relating to OAT as a component of the pharmacy’s larger QMP.
When appropriate, inform and educate patients/community regarding OAT. Information and advertisement must conform to federal and provincial legislation.
Code of Ethics
Do not forget the CoE 4 main principles
Beneficence - to benefit
Non-Maleficence - do no harm, and prevent harm from occurring
Respect for Persons
Justice
Education and Training
Managers and other professional staff are encouraged to complete one of the courses outlined in the directive by NBCP.
All pharmacy professionals must also ensure competency in dealing with adverse patient outcomes associated with OAT.
Pharmacy practitioners must be competent in dealing with opioid overdose and the use of naloxone
Managers and other professional staff are encouraged to complete one of the courses outlined in the directive by NBCP.
All pharmacy professionals must also ensure competency in dealing with adverse patient outcomes associated with OAT.
Pharmacy practitioners must be competent in dealing with opioid overdose and the use of naloxone
Pharmacists who provide injectable hydromorphone (iOAT) services: The pharmacy manager (or their delegate) plus one other pharmacist in the practice (where more than one professional is employed in the practice) must complete one of the courses listed in Appendix B of the NBCP directive as a basis for engaging in providing iOAT.
Pharmacy managers are accountable for ensuring staff competency is documented.
Equipment and Storage
Equipment monitoring, calibration and maintenance must be included in the pharmacy’s quality management program (QMP).
Measuring devices for preparation of methadone must be calibrated such that the error rate is no greater than 0.1mL therefore, the use of graduated cylinders is not acceptable for measuring of methadone. Typically, oral syringes or manual/electronic pumps that are regularly calibrated as per manufacturer specification meet this accuracy requirement.
Equipment used to prepare methadone may not be used to prepare or compound other medications. ** must be identified as dedicated only to methadone preparation.
All medications used in provision of OAT must not be visible from outside the dispensary and must be stored securely until provided to the patient
Patient Access to Pharmacy
Patients requiring daily observed dosing must access a pharmacist every day.
Where seven day per week service is not possible, pharmacy managers must accommodate patients who are not assessed as appropriate for receiving take-home 6 doses.
Options include:
Allowing patients access during short window of time to allow for daily assessment and administration
Coordinating “closed day” observed dosing with a secondary pharmacy.
Emergency planning
Documentation
Pharmacy managers are responsible for the development or adaptation of forms to document clinical activities, steps completed in the dispensing process and for setting expectations of the patient-pharmacy professional relationship.
Dispensing documentation: Pharmacists and pharmacy technicians must document each step in dispensing of OAT. Given that medication used in OUD may require dilution, and that the agents are desirable for purposes of diversion, the documentation required is greater than that required for dispensing of typical (non-OAT) oral dosage forms
Clinical documentation: Pharmacists must document patient assessment. Pharmacists or pharmacy technicians must document follow-up of patients for observed dosing or take-home dispensing
College documentation: Provision of OAT does not require authorization from the College however, the College must be notified of provision of OAT service by the pharmacy manager via online declaration within the
pharmacy’s profile. This information may be made publicly available to assist patients in finding care
Quality Management Program (QMP)
Pharmacies are required to have a QMP in place according to the regulations and the Model Standards of Practice for Pharmacists and Pharmacy Technicians in Canada
QMP documentation relating to OAT is a required component of the pharmacy’s overall QMP records.
Pharmacy managers are responsible (in collaboration with staff) for establishing/ adapting forms to meet the needs of their unique practice as well as updating, monitoring and retaining these forms
Prescription Standards and Requirements
who must prescribe OAT
Federal law and provincial policy establish prescribers of controlled substances, and are defined in:
the Controlled Drug and Substance Act(CDSA)
New Classes of Practitioners Regulations
as a class exemption for patients, practitioners and pharmacists prescribing and providing controlled substances in Canada
ALL prescription Requirements set out in federal and provincial laws, regulations and policies for drugs listed on Schedule I of the CDSA apply to therapeutic agents used in OAT.
Methadone, SROM and iOAT: MUST include start and stop dates and indicate observed dosing.
Methadone: Prescriptions for methadone must include details regarding take-home dosing schedules.
In standards, act is all listed when the prescription is given the physician will write exact start and stop dates and indicates “carries” take hom
Computer Processing
Observed doses entered into computer software and the patient’s electronic health record (EHR), but subsequently not administered to patients, must be reversed before the pharmacy closes for the day to ensure accuracy of the patient’s EHR.
This provides accurate data to other health care providers who may be caring for the patient. The prescription actual stop date (mandatory for methadone, SROM and iOAT) as expressed by the prescriber must be entered into the software. The software may require that the stop date be entered manually.
the carries must be billed on a daily basis with the stop dates
Labeling of Observing Dosing
Observed doses are exempt from labeling if prepared and immediately provided to the patient, where no opportunity for dosing or patient identification error exists.
OAT prepared ahead of time for dispensing as observed doses must be labelled, and that label must contain, at a minimum:
the name of the drug
the dose
lot number
expiry date from the stock bottle/original packaging
pharmacy team member who prepared the dose.
All labels on take-home doses must:
Comply with legal requirements
Include number of remaining part-fills, and the prescriber's stop date
Methadone
labels for take-home doses must:
Conform to practice guidelines CAMH: Pharmacist’s Guide
Include beyond-use-date (BUD) as determined by existing stability data.
Specify on the first line of the signature (sig) line “Drink the full contents of bottle (XX mg) once daily”. This ensures the dispensing record created within the patient’s EHR explicitly communicates the dose prescribed to any member of the patient care team.
Preparation of Final Dose: Buprenorphine/Naloxone
Preparation of observed and take-home dose packaging must maintain integrity of the dosage form.
Methadone Final Dose
Must be single-use daily dose regardless of patient care setting.
Calculations must be performed by a pharmacist or pharmacy technician.
Commercially available 10 mg/ml oral stock solutions must be used (a consistent manufacturer for the stock methadone solution is encouraged as dispensing of the same brand may allow patients to identify change in taste, which may signal a potential dosage error, changes in manufacturer of stock solution must be communicated to patients)
Stock solution must be diluted to total volume of approximately 100 ml with a vehicle such as Tang®, Allen’s apple juice or another crystalline juice.
Diluent juice must be refrigerated and labeled with name, date and before use date of preparation.
Must be dispensed in a light-resistant and child-resistant packaging.
Must be dispensed in a tamper-evident bottle. This facilitates take home audits and identification of misuse.
SROM Final Dose
Use SROM with 24-hour dose interval (not a product intended for 12-hour dosing)
The capsule must be opened to release the enclosed pellets for immediate consumption.
The pellets must be swallowed whole. Crushing, chewing, or dissolving slow-release oral morphine capsules or pellets can cause rapid release and absorption of a potentially fatal dose of morphine
The pellets are to be sprinkled onto a small amount of applesauce or pudding. Alternatively, pellets may be transferred into a medicine cup and ingested, followed by water to ensure all pellets have been swallowed
*is very specific
iOAT Final Dose
A pharmacist must perform the follow-up monitoring after a patient self-administers iOAT.
This function may not be performed by a pharmacy technician or unregulated member of staff.
Take-Home Doses
If the prescriber has indicated take-home doses and the pharmacist, after patient assessment, believes there is a risk to the patient of overdose, misuse or diversion, the pharmacist may refuse to dispense take-home doses, and revert to observed dosing. .
This intervention must be documented, and the prescriber must be informed.
Buprenorphine/naloxon
The maximum number of take-home doses is six.
Some patients may be sufficiently stable to allow for up to 13 take-home doses.
Take-home doses of buprenorphine-naloxone are not required to be provided in a “lock box”.
Methadone
The “lock box” should be discreet so as not to draw attention to the patient.
cash box with a key
used as child proofing
The lock box must be made of materials which will impede access to the methadone and decrease the risk of unintended ingestion by children and opioid-naïve individuals.
Softsided containers, which can be cut with a blade or scissors, are not appropriate.
Each patient must have their own identifiable “lock-box”
Patients must be informed that they may be asked at any time to appear in the pharmacy and bring with them the remainder of their take-home doses and empty bottles, in their own lock box.
This procedure, referred to as a "carry audit", may be used to assess for diversion or misuse
Take-Home Doses
SROM: To limit potential for diversion, slow-release oral morphine is only to be provided via daily observed ingestion
iOAT: To limit potential for diversion and encourage safe injection technique, iOAT is only to be provided via daily observed self-administration in accordance with policy and practice guidelines
Follow-up Adherence Monitoring Methadone
Methadone packaging may be used for illicit purposes.
To prevent diversion of methadone packaging in community pharmacy, take-home bottles must be returned to the pharmacy prior to new take-home doses being issued.
A pharmacy team member must reconcile the empty bottles returned with the number that were issued and this reconciliation must be documented.
In institutional settings, any packaging of methadone should be discarded according to institutional policy.
document that a bottle is missing and do not give them more for home tell the physician
Some patients may occasionally experience extenuating circumstances such as short-term illness or life events (e.g. public health emergencies, travel, bereavement or temporary employment demands) that present a barrier to attending a pharmacy for patient care and supply of OAT. Pharmacy professionals faced with challenging decisions regarding how to balance patient benefit with public safety should apply the Values Based Decision Model (VBDM) as described within the College’s Code of Ethics. Decision rationale must be documented within the patient profile to facilitate Intraprofessional care.
Extenuating Patient Circumstances – Potential Scenarios
Delivery:
If a patient is unable to attend the pharmacy due to extenuating circumstances OAT may be delivered, as per Health Canada.
The pharmacist must ensure the patient is assessed prior to the dose administration and follow-up monitoring subsequently occurs
Documentarian of OAT
Dispensing Documentation
Pharmacists or pharmacy technicians must record and retain documentation of the steps in preparation and provision of OAT as per standards of practice and regulations.
Pharmacy professionals (community pharmacy only) must document the return of take-home methadone bottles.
Clinical Documentation
Pharmacists must record and retain documentation of their patient assessment, clinical decision-making (including documentation of care decisions made in response to exceptional circumstances)
and follow-up monitoring as per established standards of practice and regulation
anything done and is beyond pharmacy documentation has to be done and possible sent to the physician in the computer system in the patients file
Inter/Intraprofessional Documentation
Prescription Monitoring Program (PMP) Through electronic health record (EHR)
The dose and the date of last observed dose must be documented in the PMP, if a dose is not administered as expected, before end of the day, the pharmacy must ensure the dispensing record is amended to ensure accuracy (use alternate means of communication (telephone, secure electronic messaging or in-person discussion) to ensure safe patient care.
Inter/Intraprofessional documentation of the provision of OAT is vital to the safe and effective care of patients as they transition between care environments and providers.
Transition of Custody
Pharmacy professionals may collaborate with other health care professionals to provide patient-specific prepared and labeled doses of OAT to an externally located patient care site. This is considered a transfer of custody.
The following professionals can be involved in administering OAT to patients and may possess patient doses of OAT:
Another pharmacist
Hospital employee authorized to receive substances listed within Schedule 1 of the CDSA
Medical practitioner (physician)
Nurses practicing as per Health Canada’s Subsection 56(1) (Class Exemption for Nurses Providing Health Care at a Community Health Facility)
Nurse practitioners as per (Section 2 of the New Classes of Practitioners Regulations to Controlled Drugs and Substances Act)
Pharmacy professionals must facilitate patient and community safety as best possible by supporting these providers to:
Confirm receipt of doses through a signature of the health care professional
Accomplish dose adjustments through provision of a new pharmacy- prepared dose
Avoid alteration of patient-labeled doses
Avoid altering dose volumes for administration
Prohibit use of a dose intended for one patient by another
Return unused patient doses within 72 hours
OAT Quality Management Program (QMP)
Education and Training Documentation: Pharmacy professionals must document within the pharmacy’s QMP that they have established and maintained competency for providing OAT for patients with OUD
Medication Incidents (Errors and Near-Misses) and Continuous Quality Improvement (CQI)
All medication incidents must be addressed according to the College’s “Practice Directive: Mandatory
Medication Incident Reporting (MMIR)” , the NAPRA Model Standards of Practice for Pharmacists and Pharmacy Technicians in Canada.
**Please refer to CAMH: Pharmacist’s Guide for specific management of OAT medication incident
OAT Quality Management Program
Equipment Provisions: ongoing monitoring of equipment used in providing OAT are outlined in the College’s Expectations for a Quality Management Program
Facilities Provisions: ongoing monitoring of the physical facilities where OAT is provided are outlined in the College’s Expectations for a Quality Management Program
 Knowt
Knowt