
Unit 9- Redox/Electrochemistry
DO NOT CLICK FLASHCARDS FROM HERE (OR STUDY) Click Here.
* Reminder: when an atom has a positive charge, there are less electrons due to them being negatively charged. Same for when atoms have negative charges, there it has more electrons.
* Any code blocks ( ) will represent subscripts. Ex: Carbon Dioxide= CO2
Oxidation States/Numbers:
→ The charge of elements. (The ones on the upper right hand corner of the periodic table for each element)
Since most elements have more than one oxidation state, you can calculate the exact ones for each element in a specific reaction or compound.
An element could have an oxidation state not written in the periodic table.
Rule for calculating Oxidation states:
Oxygen is always 2-, with two exceptions
H
2O2, O is 1-OF
2, O is 2+
Hydrogen and most group 1 ions are almost always 1+ (Can also be written as +)
Halogens (group 7 ions) will almost always be 1- (Can also be written as -)
Elements on their own (in a reaction) are always 0, unless a charge is written on the element symbol.
Diatomic elements are always 0.
In a compound the sum of the oxidation states must equal zero
If it’s a polyatomic ion, the sum must equal the charge of the compound.
Examples of calculations:
BeO
Since O is always 2-, the oxidation state of Be must be 2+, due to the fact that the sum of the numbers should be zero in neutral compounds
Redox reactions:
→ Redox is short for “Oxidation Reduction”
Oxidation and Reduction:
Oxidation → The lose of electrons by an atom (an atom becoming more positive)
Reduction →The gaining of electrons by an atom (an atom becoming more negative)
Think of it as the oxidation number is reducing, rather than the amount of electrons.
A way to remember is LEO the lion says GER
LEO stand for “Loosing electrons is Oxidation”
GER stand for “Gaining electrons is Reduction”
The Reactions:
In redox reactions one element is losing electrons (Oxidizing), while another is gaining those electrons (Reducing). These two thing happen simultaneously
In these reaction the element which is oxidized (lost electrons), is known as the Reducing Agent.
The Element which is reduced (gained electrons), is known as the Oxidizing Agent.
The oxidation states/charges of elements will change from the reactants to the products.
For Example:
Na + Cl → NaCl, is a redox reaction, since Na and Cl are both neutral on the reactants side, but have charges on the products.
Na on the products side has a charge of 1+, and Cl has a charge of 1-.
Na lost an electrons so it was oxidized (was the reducing agent)
Cl gained an electron so it was reduced (was the oxidizing agent)
If a reaction does not have both oxidation and reduction happening it is not a redox reaction.
Half Reactions:
→ Redox reactions must be balanced for BOTH mass and CHARGE. To do this we use half reactions to show both processes (Oxidation and Reduction).
A redox reaction can be split in to two half reactions, one for the element being oxidized, and the other for the element being reducded.
If there are any ions or elements where the charge doesn’t change, they are left out of these half reactions.
These are known as spectator ions.
In the half reaction we also show the electrons, represented by "e-”.
Before writing half reaction make sure the mass is balanced (same amount of each element on both sides)
Then when the half reaction are written, there should be the same amount of “e-” in both halves.
If not then you need to manipulate them so they do.
When the two half reaction are added together you can cancel out the “e-”, and you should be left with a balanced version of the original reaction
Some reaction are already balanced for both.
Examples:
Na + Cl → NaCl, same as above.
The Oxidation half reaction would be: Na→ Na^1+ + e-
The Reduction half reaction would be: Cl + e- → Cl^1-
Charges are usually written as exponents/superscripts.
Notice the e- lost by Na, is the same e- gained by Cl. (This reaction is already balanced)
(Insert picture of 3. on pg 12)
Simultaneous Reactions and Table J:
On Table J, metals that are higher up will be oxidized
And the lower ones will be reduced.
So if K and Ca were in a redox reaction, K should oxidize since it is higher up than Ca.
If the metal that gets oxidized is lower, than the metal being reduced than the reaction will NOT be spontaneous. (There are scenarios where this could happen)
If the metal that is oxidized is higher than the one being reduced, the reaction WILL be spontaneous.
Cells/Batteries:
→ There are two types of cells/Batteries: Galvanic/Voltaic cells, and Electrolytic cells. Though they work differently they do have some of the same parts. (They are both referred to as electrochemical cells)
There are two electrodes in each battery. The electrons are where the electrons are oxidized and reduced.
Anode → The electrode where oxidation will occur in the cell.
Similar to an anion, because it is the negative end of a batterie.
Cathode → The electrode where reduction will occur in the cell.
Like a Cation, it is the positive end.
You can remember these by using "An Ox”, and "Red Cat”
An Ox, stands for Anode is Oxidation
Red Cat, stands for Cathode is Reduction.
The Anode and the Cathode are connected by a wire, where the electrons will flow through.
Voltaic/Galvanic Cells:
→ Both names mean the same thing.
These cells produce energy
The Anode and Cathode are separated, either in two separate containers filled with salt solutions, or by a membrane in the same container.
There is still a wire for connection.
Electrons will flow from the Anode to the Cathode.
From the electrode which loses electrons to the one which gains electrons.
A salt bridge also connects the two solutions in the beakers. This helps maintain equilibrium by moving the icons around.
The ions will flow the opposite way as the electrons. from the cathode side to the anode side.
As the cell goes through Oxidation and Reduction the Anode will lose mass and the Cathode will gain mass.
This is because the Anode loses atoms to the solution as they become ions from losing their electrons. (They fall off of the Anode)
The Cathode gains those electrons, which the ions in the solution will accept and they will then become neutral atoms and attach to the Cathode.
It converts chemical energy to electrical energy.
Example: (Picture)
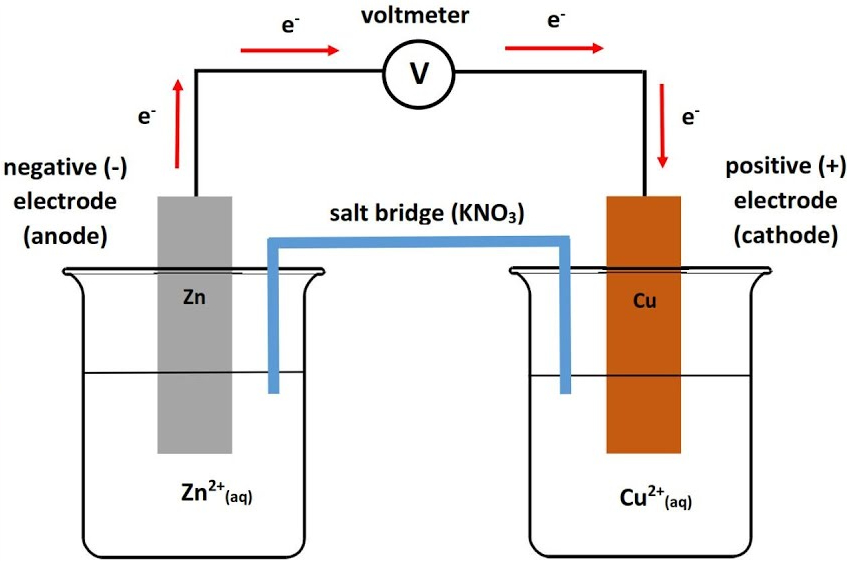
Zn is the anode because it is higher up on Table J, than Cu, so it will oxidize.
The electrons are flowing from Zn (anode) to the Cu (Cathode)
The salt bridge is carrying ions from the Cu side to the Zn side.
Over time the anode will lose Zn atoms as they become ions, and the cathode will gain Cu atoms as they become neutral.
Electrolytic Cells:
These cells require external energy to work. (Usually a battery)
This is because it is forcing a spontaneous reaction to happen.
The Anode and Cathode are switched. So the one being oxidized is the one which is usually reduced in a Galvanic cell.
They convert electrical energy into chemical energy
There is no salt bridge since the entire cell is in one container.
These cells are usually used to cat items so the cathode is usually an item like a key or some type of cutlery
This process is known as electroplating.
Example: (Picture)
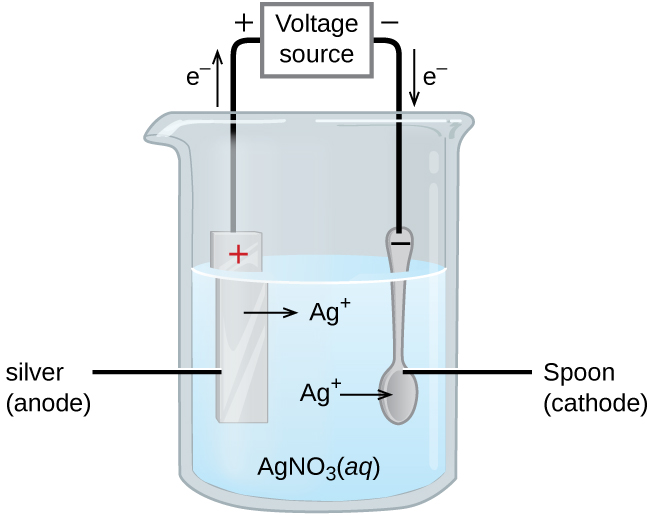
The Silver anode is losing electrons and those electrons are being transferred to the spoon.
The spoon accepts them and then the Ag+ ions in the solution take the electrons and attach to the spoon.
This will plate the spoon in silver. Increasing the mass of the spoon.
The voltage source gives electricity to force the nonspontaneous transfer of electrons
Next Unit: Organic Chemistry
Unit 9- Redox/Electrochemistry
DO NOT CLICK FLASHCARDS FROM HERE (OR STUDY) Click Here.
* Reminder: when an atom has a positive charge, there are less electrons due to them being negatively charged. Same for when atoms have negative charges, there it has more electrons.
* Any code blocks ( ) will represent subscripts. Ex: Carbon Dioxide= CO2
Oxidation States/Numbers:
→ The charge of elements. (The ones on the upper right hand corner of the periodic table for each element)
Since most elements have more than one oxidation state, you can calculate the exact ones for each element in a specific reaction or compound.
An element could have an oxidation state not written in the periodic table.
Rule for calculating Oxidation states:
Oxygen is always 2-, with two exceptions
H
2O2, O is 1-OF
2, O is 2+
Hydrogen and most group 1 ions are almost always 1+ (Can also be written as +)
Halogens (group 7 ions) will almost always be 1- (Can also be written as -)
Elements on their own (in a reaction) are always 0, unless a charge is written on the element symbol.
Diatomic elements are always 0.
In a compound the sum of the oxidation states must equal zero
If it’s a polyatomic ion, the sum must equal the charge of the compound.
Examples of calculations:
BeO
Since O is always 2-, the oxidation state of Be must be 2+, due to the fact that the sum of the numbers should be zero in neutral compounds
Redox reactions:
→ Redox is short for “Oxidation Reduction”
Oxidation and Reduction:
Oxidation → The lose of electrons by an atom (an atom becoming more positive)
Reduction →The gaining of electrons by an atom (an atom becoming more negative)
Think of it as the oxidation number is reducing, rather than the amount of electrons.
A way to remember is LEO the lion says GER
LEO stand for “Loosing electrons is Oxidation”
GER stand for “Gaining electrons is Reduction”
The Reactions:
In redox reactions one element is losing electrons (Oxidizing), while another is gaining those electrons (Reducing). These two thing happen simultaneously
In these reaction the element which is oxidized (lost electrons), is known as the Reducing Agent.
The Element which is reduced (gained electrons), is known as the Oxidizing Agent.
The oxidation states/charges of elements will change from the reactants to the products.
For Example:
Na + Cl → NaCl, is a redox reaction, since Na and Cl are both neutral on the reactants side, but have charges on the products.
Na on the products side has a charge of 1+, and Cl has a charge of 1-.
Na lost an electrons so it was oxidized (was the reducing agent)
Cl gained an electron so it was reduced (was the oxidizing agent)
If a reaction does not have both oxidation and reduction happening it is not a redox reaction.
Half Reactions:
→ Redox reactions must be balanced for BOTH mass and CHARGE. To do this we use half reactions to show both processes (Oxidation and Reduction).
A redox reaction can be split in to two half reactions, one for the element being oxidized, and the other for the element being reducded.
If there are any ions or elements where the charge doesn’t change, they are left out of these half reactions.
These are known as spectator ions.
In the half reaction we also show the electrons, represented by "e-”.
Before writing half reaction make sure the mass is balanced (same amount of each element on both sides)
Then when the half reaction are written, there should be the same amount of “e-” in both halves.
If not then you need to manipulate them so they do.
When the two half reaction are added together you can cancel out the “e-”, and you should be left with a balanced version of the original reaction
Some reaction are already balanced for both.
Examples:
Na + Cl → NaCl, same as above.
The Oxidation half reaction would be: Na→ Na^1+ + e-
The Reduction half reaction would be: Cl + e- → Cl^1-
Charges are usually written as exponents/superscripts.
Notice the e- lost by Na, is the same e- gained by Cl. (This reaction is already balanced)
(Insert picture of 3. on pg 12)
Simultaneous Reactions and Table J:
On Table J, metals that are higher up will be oxidized
And the lower ones will be reduced.
So if K and Ca were in a redox reaction, K should oxidize since it is higher up than Ca.
If the metal that gets oxidized is lower, than the metal being reduced than the reaction will NOT be spontaneous. (There are scenarios where this could happen)
If the metal that is oxidized is higher than the one being reduced, the reaction WILL be spontaneous.
Cells/Batteries:
→ There are two types of cells/Batteries: Galvanic/Voltaic cells, and Electrolytic cells. Though they work differently they do have some of the same parts. (They are both referred to as electrochemical cells)
There are two electrodes in each battery. The electrons are where the electrons are oxidized and reduced.
Anode → The electrode where oxidation will occur in the cell.
Similar to an anion, because it is the negative end of a batterie.
Cathode → The electrode where reduction will occur in the cell.
Like a Cation, it is the positive end.
You can remember these by using "An Ox”, and "Red Cat”
An Ox, stands for Anode is Oxidation
Red Cat, stands for Cathode is Reduction.
The Anode and the Cathode are connected by a wire, where the electrons will flow through.
Voltaic/Galvanic Cells:
→ Both names mean the same thing.
These cells produce energy
The Anode and Cathode are separated, either in two separate containers filled with salt solutions, or by a membrane in the same container.
There is still a wire for connection.
Electrons will flow from the Anode to the Cathode.
From the electrode which loses electrons to the one which gains electrons.
A salt bridge also connects the two solutions in the beakers. This helps maintain equilibrium by moving the icons around.
The ions will flow the opposite way as the electrons. from the cathode side to the anode side.
As the cell goes through Oxidation and Reduction the Anode will lose mass and the Cathode will gain mass.
This is because the Anode loses atoms to the solution as they become ions from losing their electrons. (They fall off of the Anode)
The Cathode gains those electrons, which the ions in the solution will accept and they will then become neutral atoms and attach to the Cathode.
It converts chemical energy to electrical energy.
Example: (Picture)

Zn is the anode because it is higher up on Table J, than Cu, so it will oxidize.
The electrons are flowing from Zn (anode) to the Cu (Cathode)
The salt bridge is carrying ions from the Cu side to the Zn side.
Over time the anode will lose Zn atoms as they become ions, and the cathode will gain Cu atoms as they become neutral.
Electrolytic Cells:
These cells require external energy to work. (Usually a battery)
This is because it is forcing a spontaneous reaction to happen.
The Anode and Cathode are switched. So the one being oxidized is the one which is usually reduced in a Galvanic cell.
They convert electrical energy into chemical energy
There is no salt bridge since the entire cell is in one container.
These cells are usually used to cat items so the cathode is usually an item like a key or some type of cutlery
This process is known as electroplating.
Example: (Picture)

The Silver anode is losing electrons and those electrons are being transferred to the spoon.
The spoon accepts them and then the Ag+ ions in the solution take the electrons and attach to the spoon.
This will plate the spoon in silver. Increasing the mass of the spoon.
The voltage source gives electricity to force the nonspontaneous transfer of electrons
 Knowt
Knowt
