
2: Chemistry Comes Alive
Matter and Energy
Matter
Matter: It is anything that occupies space and has mass.
The mass of an object is equal to the actual amount of matter in the object, and it remains constant wherever the object is.
Weight varies with gravity.
Solid: Has a definite shape and volume.
Liquid: Has a definite volume, but they conform to the shape of its container.
Gas: Has neither a definite shape nor a definite volume.
Energy
Energy: The capacity to do work, or to put matter into motion.
The greater the work done, the more energy is used doing it.
Kinetic energy: Energy in motion/action.
Potential energy: Stored energy, an inactive energy that has the capability to do work, but is not presently doing so.
Matter is the substance and energy is the mover of the substance.
Forms of Energy
Chemical energy: It is the form stored in the bonds of chemical substances.
Electrical energy: Energy that results from the movement of charged particles.
Mechanical energy: Energy directly involved in moving matter.
Radiant energy: Also called electromagnetic energy — Energy that travels in waves.
Composition of Matter: Atoms and Elements
Elements: Unique substances that cannot be broken down into simpler substances by ordinary chemical methods.
There are 118 elements that are currently recognized.
92 occur in nature and the rest are artificially made.
Periodic Table: A table that provides a more complete listing of the known elements and helps to explain the properties of each element that make it react as it does with other elements.
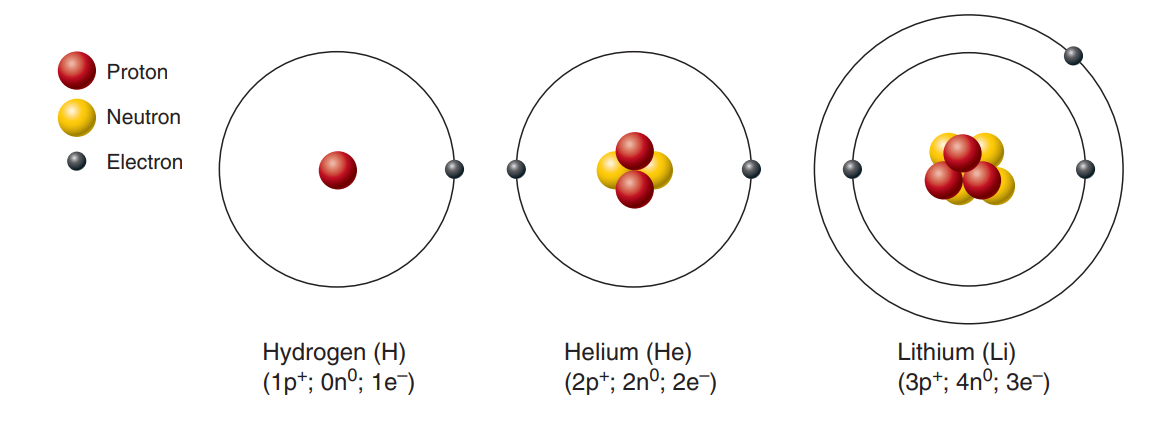
Atoms: Building blocks or, more or less identical particles of which an element is composed of.
The smallest atoms are less than 0.1 nanometer (nm) in diameter, and the largest are only about five times as large.
Physical properties of atoms: Those we can detect with our senses or measure.
Chemical properties of atoms: Pertain to the way atoms interact with other atoms (bonding behavior) and account for the facts that iron rusts, animals can digest their food, and so on.
Atomic Symbol: A one- or two-letter chemical shorthand used to designate each element, usually the first letter(s) of the element’s name.
Atomic Structure
An atom’s subatomic particles differ in mass, electrical charge, and position in the atom.
An atom has a central nucleus containing protons and neutrons tightly bound together.
Proton: A subatomic particle that bears a positive electrical charge.
Neutron: A subatomic particle that is neutral.
Electrons: A subatomic particle that bears a negative charge equal in strength to the positive charge of the proton.
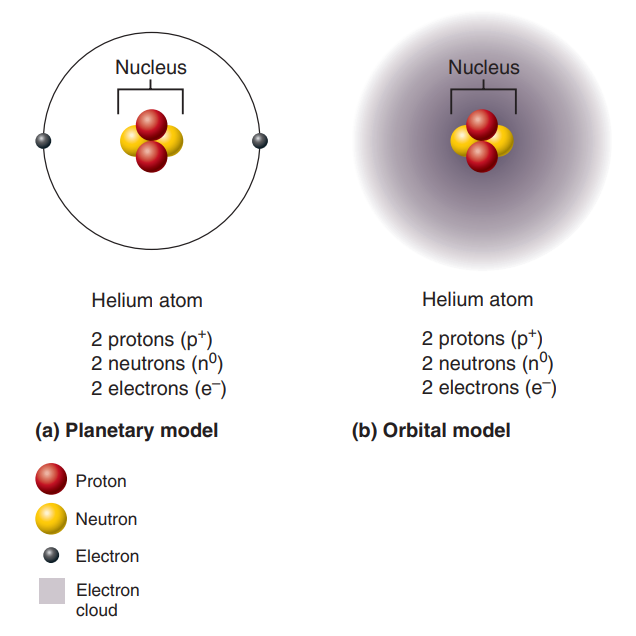
Planetary Model: A simplified model of atomic structure.
Orbitals: Regions around the nucleus in which a given electron or electron pair is likely to be found most of the time.
Orbital Model: A more modern model of atomic structure, which is more useful for predicting the chemical behavior of atoms.
Common Elements Composing the Human Body
Element | % Body Mass | Function |
|---|---|---|
Oxygen (O) | 65.0 % | Needed for the production of ATP |
Carbon (C) | 18.5 % | A primary component of all organic molecules. |
Hydrogen (H) | 9.5 % | As an ion, it influences the pH of body fluids. |
Nitrogen (N) | 3.2 % | A component of proteins and nucleic acids. |
Calcium (Ca) | 1.5 % | Its ion form is required for muscle contraction, conduction of nerve impulses, and blood clotting. |
Phosphorus (P) | 1.0 % | Part of calcium phosphate salts in bones and teeth. |
Potassium (K) | 0.4 % | Necessary for conduction of nerve impulses and muscle contraction. |
Sulfur (S) | 0.3 % | Component of proteins, particularly muscle proteins |
Sodium (Na) | 0.2 % | Important for water balance, conduction of nerve impulses, and muscle contraction. |
Chlorine (Cl) | 0.2 % | Its ion is the most abundant negative ion (anion) in extracellular fluids. |
Magnesium (Mg) | 0.1 % | Also an important cofactor in a number of metabolic reactions. |
Iodine (I) | 0.1 % | Needed to make functional thyroid hormones. |
Iron (Fe) | 0.1 % | Component of hemoglobin and some enzymes. |
Identifying Elements
The atomic number of any atom is equal to the number of protons in its nucleus and is written as a subscript to the left of its atomic symbol.
The number of protons is always equal to the number of electrons in an atom, so the atomic number indirectly tells us the number of electrons in the atom as well.
The mass number of an atom is the sum of the masses of its protons and neutrons.
It is usually indicated by a superscript to the left of the atomic symbol.
Isotopes: A structural variation that all known elements have — it has the same number of protons, but differ in the number of neutrons they contain.
Atomic weight: An average of the relative weights (mass numbers) of all the isotopes of an element, taking into account their relative abundance in nature.
As a rule, the atomic weight of an element is approximately equal to the mass number of its most abundant isotope.
Radioactivity: The process of atomic decay.
Radioisotopes: Isotopes that exhibit the behaviour of radioactivity.
Half-Life: The time required for a radioisotope to lose one-half of its activity.
How Matter Is Combined: Molecules and Mixtures
Molecules and Compounds
Molecule: A combination of two or more atoms held together by chemical bonds.
If two or more atoms of the same element combine, the resulting substance is called a molecule of that element.
When two or more different kinds of atoms bind, they form molecules of a compound.
Mixture
Mixtures: These are substances composed of two or more components physically intermixed.
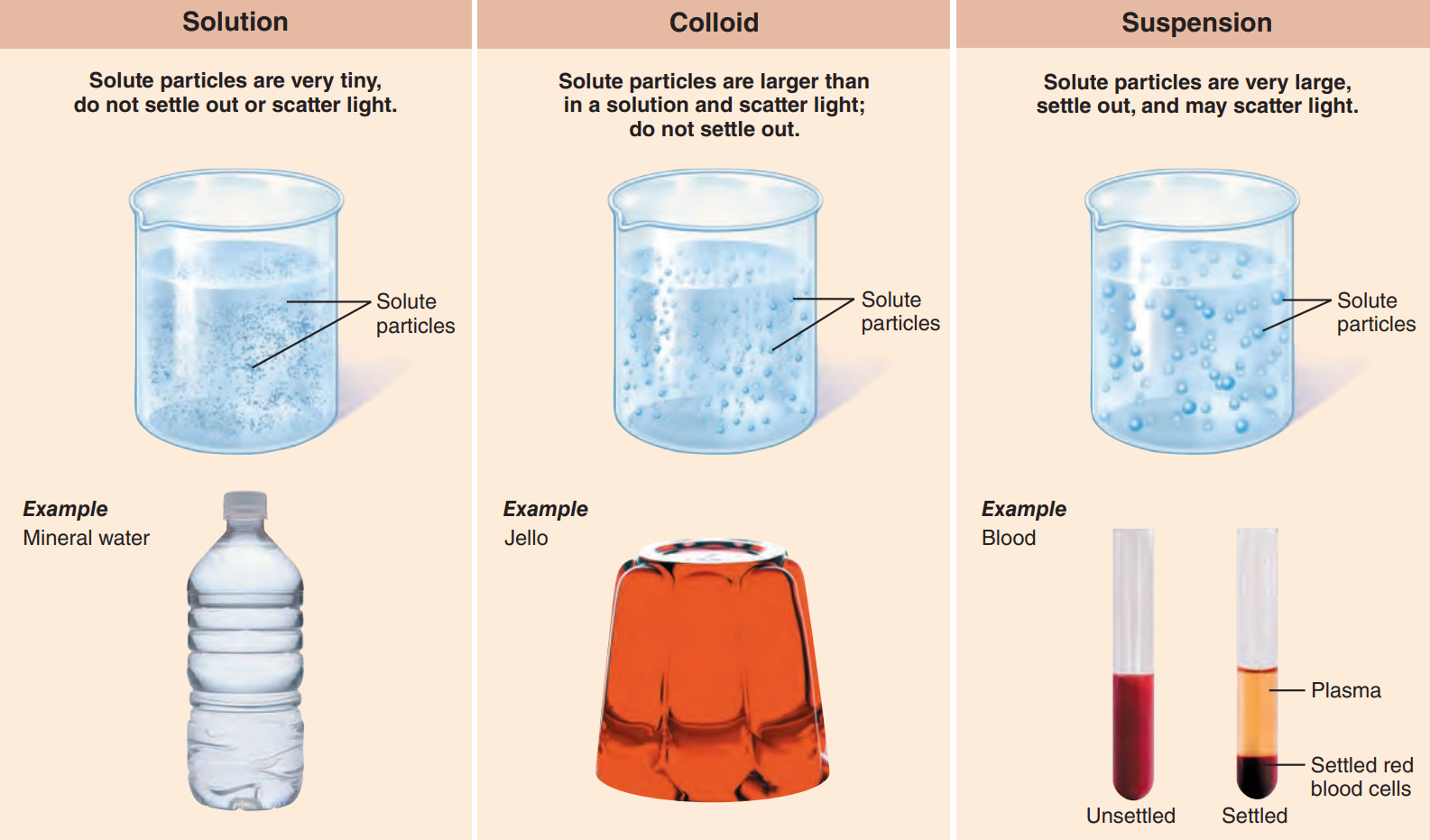
Solutions: These are homogeneous mixtures of components that may be gases, liquids, or solids.
Solvent: Substance present in greatest amounts.
Solute: Substance present in smaller amounts.
Water: The body’s chief solvent.
Most solutions in the body are true solutions containing gases, liquids, or solids dissolved in water. These solutions are usually transparent.
Solutions used in a college laboratory or a hospital are often described in terms of the percent of the solute in the total solution.
Milligrams per deciliter (mg/dl) is another common concentration measurement.
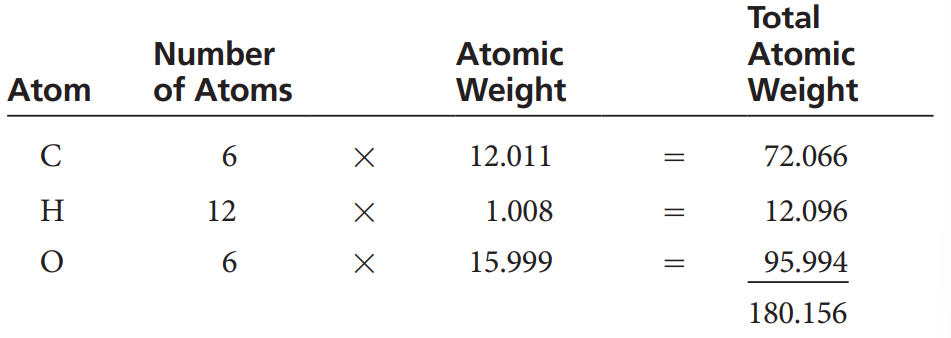
Molarity: A way to express the concentration of a solution — or moles per liter. A more complicated method but much more useful.
A mole of any element or compound is equal to its atomic weight or molecular weight weighed out in grams.
Avogadro’s number: One mole of any substance always contains exactly the same number of solute particles, that is, 6.02 x 10^23.
Colloid: Also called emulsion, — are heterogeneous mixtures, which means that their composition is dissimilar in different areas of the mixture.
It often appear translucent or milky and although the solute particles are larger than those in true solutions, they still do not settle out.
Sol-gel transformation: A unique property of colloid that change reversibly from a fluid (sol) state to a more solid (gel) state.
Cytosol: The semifluid material in living cells, is also a colloid, largely because of its dispersed proteins.
Suspensions: These are heterogeneous mixtures with large, often visible solutes that tend to settle out.
Chemical Bonds
Electrons forming the electron cloud around the nucleus of an atom occupy regions of space called electron shells that consecutively surround the atomic nucleus.
The atoms known so far can have electrons in seven shells, but the actual number of electron shells occupied in a given atom depends on the number of electrons that atom has.
The attraction between the positively charged nucleus and negatively charged electrons is greatest when electrons are closest to the nucleus and falls off with increasing distance.
This statement explains why electrons farthest from the nucleus
have the greatest potential energy, and
are most likely to interact chemically with other atoms.
When we consider bonding behavior, the only electrons that are important are those in the atom’s outermost energy level. Inner electrons usually do not take part in bonding because they are more tightly held by the atomic nucleus.
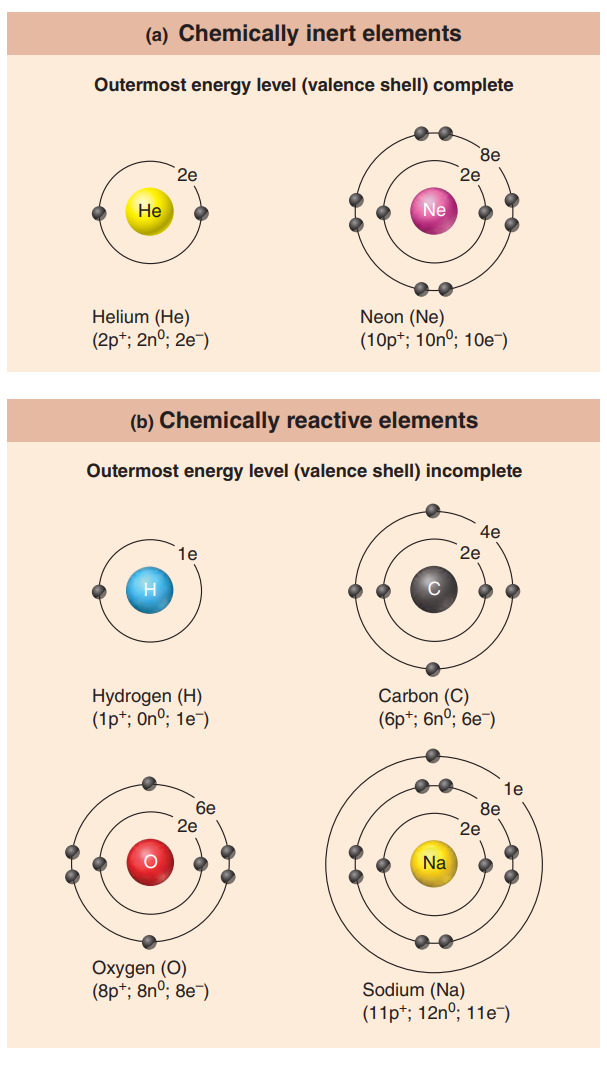
When the outermost energy level of an atom is filled to capacity or contains eight electrons, the atom is stable.
Such atoms are chemically inert, that is, unreactive.
A group of elements called the noble gases, which include helium and neon, typify this condition.
Atoms in which the outermost energy level contains fewer than eight electrons tend to gain, lose, or share electrons with other atoms to achieve stability.
The number of electrons that can participate in bonding is still limited to a total of eight.
Valence Shell: It indicates an atom’s outermost energy level or that portion of it containing the electrons that are chemically reactive.
The key to chemical reactivity is the octet rule or rule of eights.
Types of Chemical Bonds
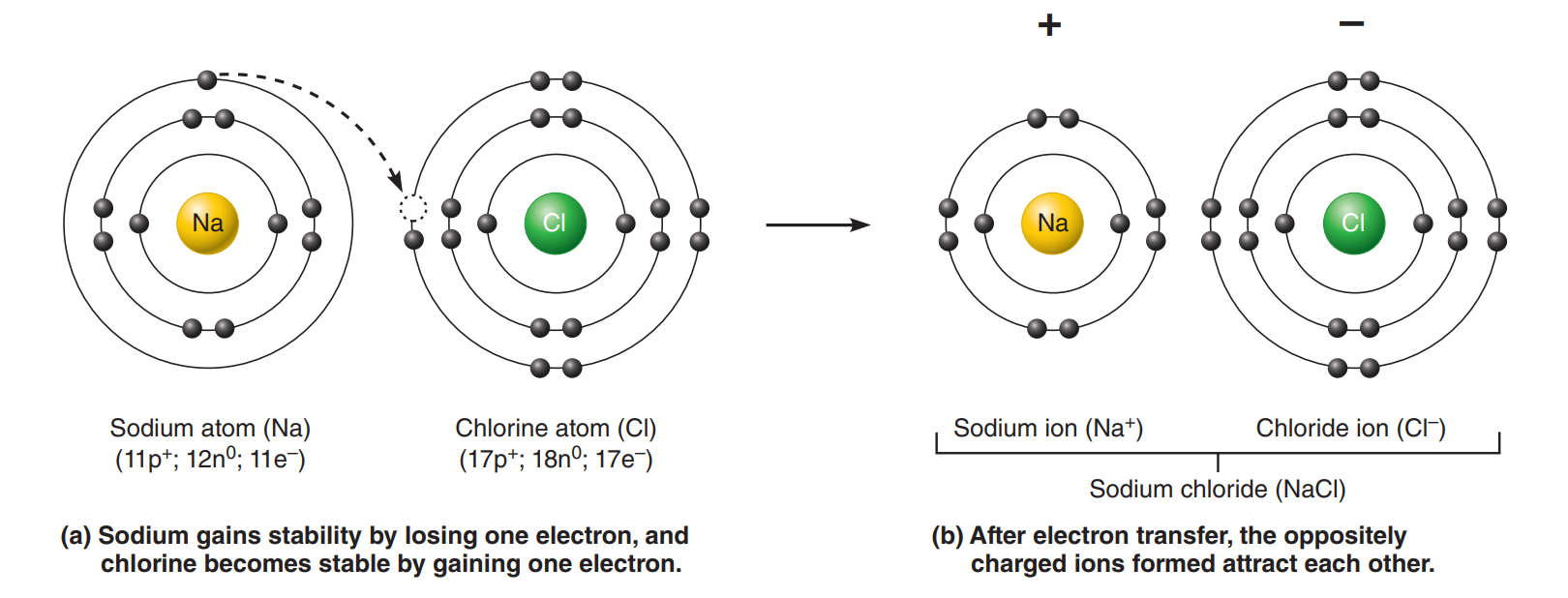
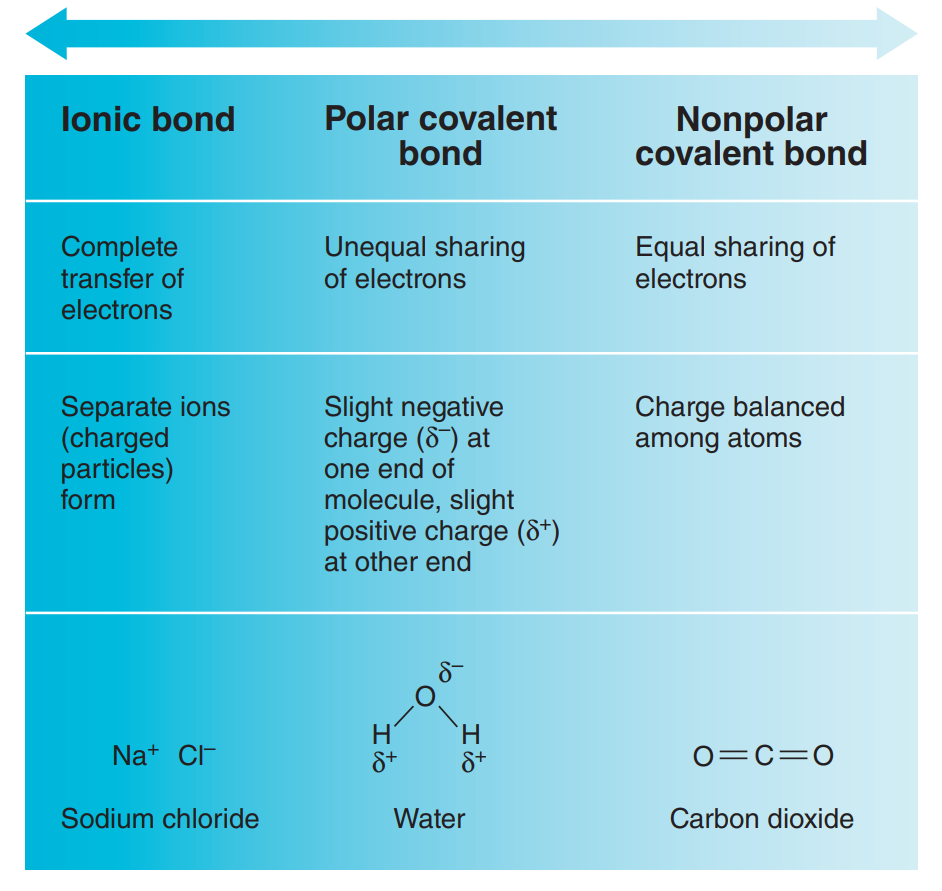
Ionic Bond
Ionic Bond: A chemical bond between atoms formed by the transfer of one or more electrons from one atom to the other.
Ions: An atom or molecule with a net electric charge due to the loss or gain of one or more electrons.
Electron acceptor: The atom that gains one or more electrons.
Anion: A net negative charge.
Electron donor: The atom that loses electrons.
Cation: A net positive charge.
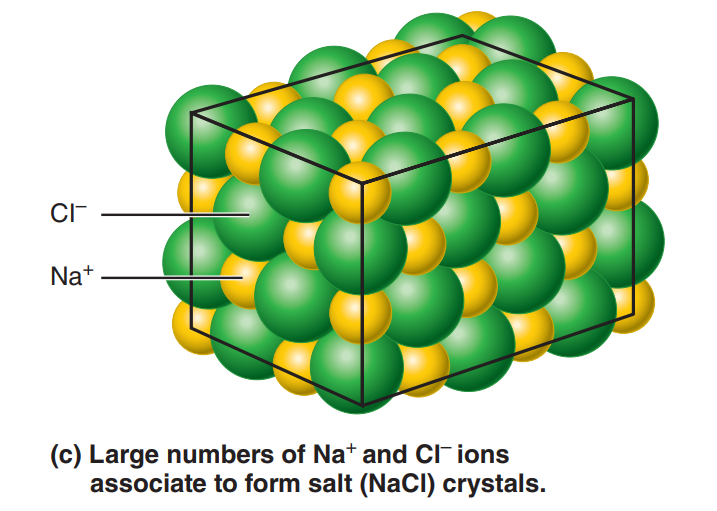
Both anions and cations are formed whenever electron transfer between atoms occurs. Because opposite charges attract, these ions tend to stay close together, resulting in an ionic bond.
Ionic bonds are commonly formed between atoms with one or two valence shell electrons and atoms with seven valence shell electrons .
Crystals: Large arrays of cations and anions held together by ionic bonds.
Covalent Bond
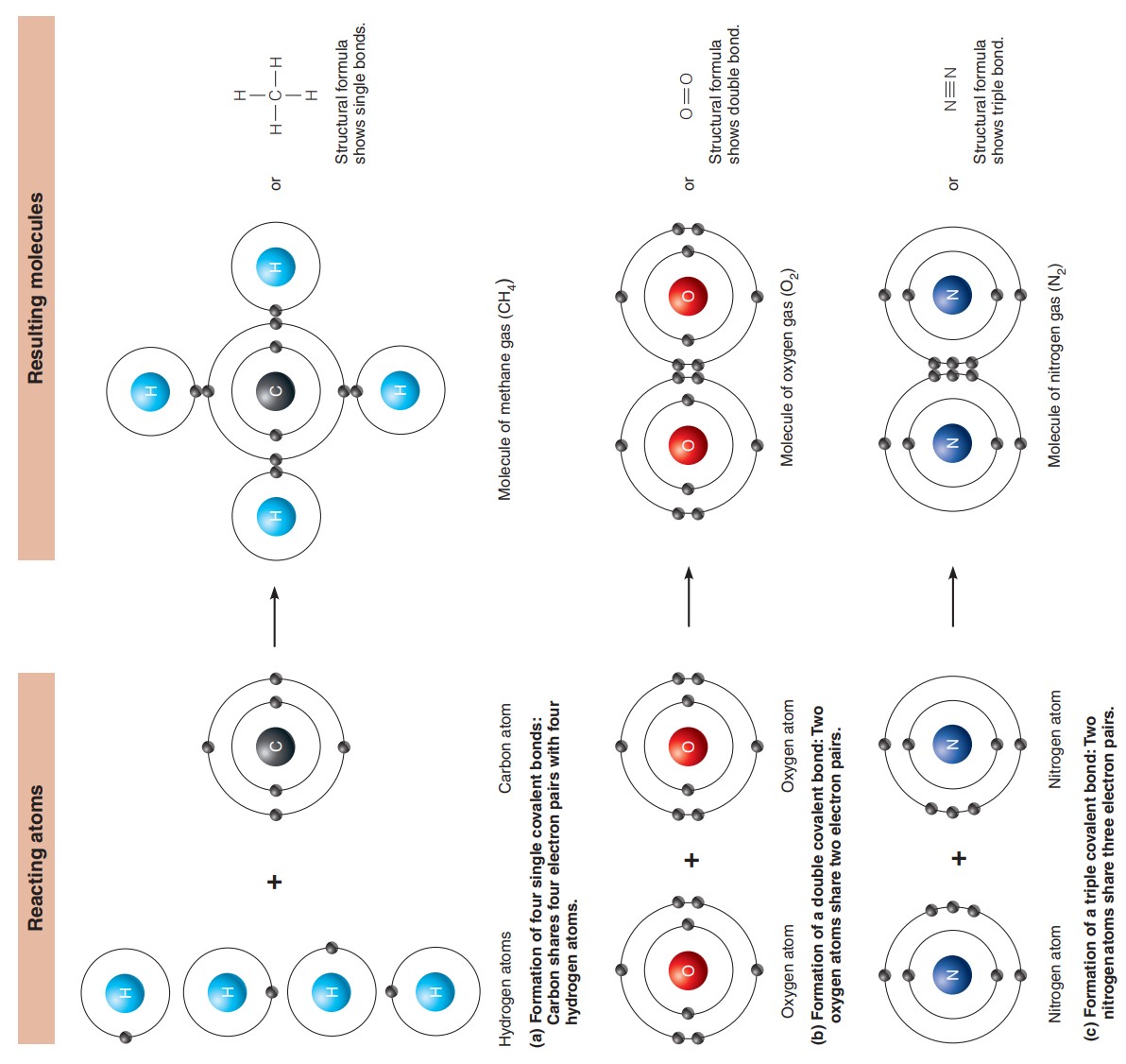
Covalent Bond: Electron sharing produces molecules in which the shared electrons occupy a single orbital common to both atoms.
Hydrogen with its single electron can fill its only shell (shell 1) by sharing a pair of electrons with another atom.
When it shares with another hydrogen atom, a molecule of hydrogen gas is formed.
The shared electron pair orbits around the molecule as a whole, satisfying the stability needs of each atom.
When two atoms share one pair of electrons, a single covalent bond is formed (indicated by a single line connecting the atoms, such as H¬H).
Nonpolar molecules: The molecules formed are electrically balanced. There has no separation of charge, so no positive or negative poles are formed.
Polar molecule: A molecule containing polar bonds where the sum of all the bond's dipole moments is not zero.
Electronegativity: The tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond.
Electropositivity: A measure of an element's ability to donate electrons, and therefore form positive ions; thus, it is antipode to electronegativity.
Dipole: Any molecule or radical that has delocalised positive and negative charges
Hydrogen Bond
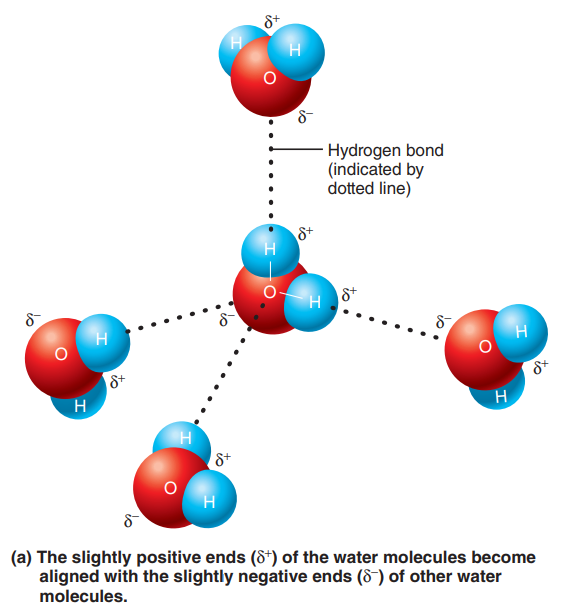
Hydrogen bonds: It is formed when a hydrogen atom, already covalently linked to one electronegative atom (usually nitrogen or oxygen), is attracted by another electron-hungry atom, so that a “bridge” forms between them.
Hydrogen bonding is common between dipoles such as water molecules, because the slightly negative oxygen atoms of one molecule attract the slightly positive hydrogen atoms of other molecules.
Hydrogen bonding is responsible for the tendency of water molecules to cling together and form films, referred to as surface tension.
Although hydrogen bonds are too weak to bind atoms together to form molecules, they are important intramolecular bonds (literally, bonds within molecules), which hold different parts of a single large molecule in a specific three-dimensional shape.
Chemical Reactions
Chemical Reaction: It occurs whenever chemical bonds are formed, rearranged, or broken.
Chemical Equations
We can write chemical reactions in symbolic form as chemical equations. For example, we indicate the joining of two hydrogen atoms to form hydrogen gas as
H + H → H2 (hydrogen gas)and the combining of four hydrogen atoms and one carbon atom to form methane as
4H + C → CH4 (methane)
A number written as a subscript indicates that the atoms are joined by chemical bonds. But a number written as a prefix denotes the number of unjoined atoms or molecules.
CH4 reveals that four hydrogen atoms are bonded together with carbon to form the methane molecule, but 4H signifies four unjoined hydrogen atoms.
A chemical equation is like a sentence describing what happens in a reaction.
It contains the following information:
reactants, the number and kinds of reacting substances,
the chemical composition of the product(s)
in balanced equations, the relative proportion of each reactant and product.
Patterns of Chemical Reactions
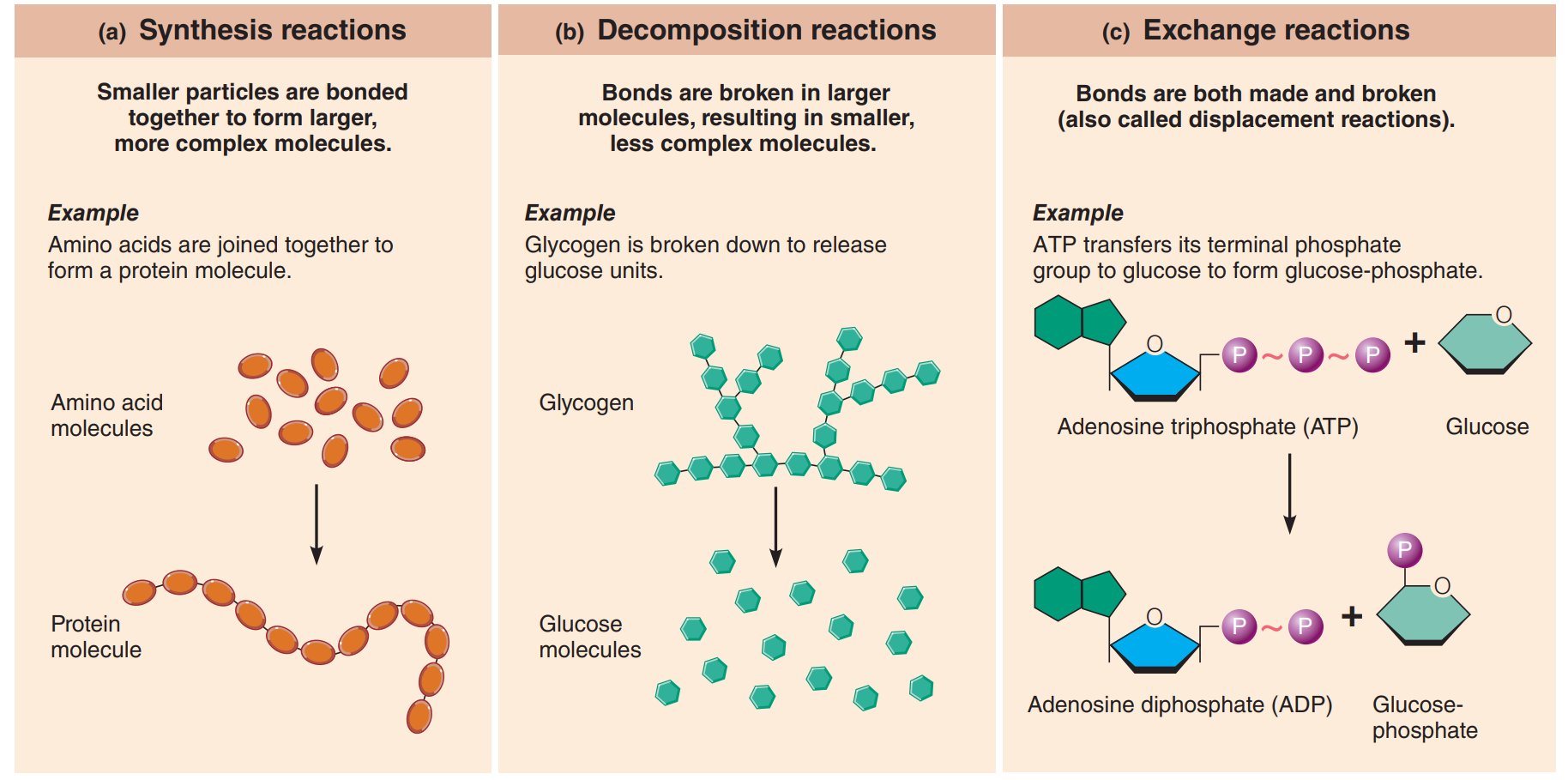
Synthesis Reaction: Also known as a Combination reaction, it occurs when atoms or molecules combine to form a larger, more complex molecule.
A + B → AB
Bond formation.
Basis of constructive, or anabolic activities in body cells, such as joining small molecules called amino acids into large protein molecules.
Decomposition Reaction: This occurs when a molecule is broken down into smaller molecules or its constituent atoms.
AB → A + B
Bonds are broken.
Underlie all degradative, or catabolic, processes in body cells.
Exchange Reaction: Also known as the displacement reaction, it involve both synthesis and decomposition.
AB + C → AC + B and AB + CD → AD + CB
Bonds are both made and broken
Parts of the reactant molecules change partners, so to speak, producing different product molecules.
Occurs when ATP reacts with glucose and transfers its end phosphate group to glucose, forming glucose-phosphate. ATP becomes ADP, too.
Oxidation-Reduction Reaction: Also known as a redox reaction.
Decomposition reactions are the basis of all reactions in which food fuels are broken down for energy.
Here, electrons are exchanged between the reactants.
Electron donors — reactant losing the electrons; is said to be oxidised.
Electron acceptors — reactant taking up the transferred electrons; is said to be reduced.
This reaction also occurs when the ionic compound is formed.
Not all of these kinds of reactions involve the complete transfer of electrons — some change the pattern of electron sharing in covalent bonds.
Cellular Respiration: A series of chemical reactions that break down glucose to produce ATP, which may be used as energy to power many reactions throughout the body.
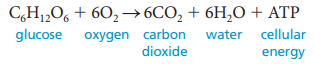
Energy Flow in Chemical Reactions
Exergonic reactions: Reactions that release energy.
Yield products with less energy than the initial reactants, along with energy that can be harvested for other uses.
Ex.: Catabolic and oxidative reactions
Endergonic reactions: Reactions that absorb energy.
Yield products that contain more potential energy in their chemical bonds than did the reactants
Ex.: Anabolic reactions
Reversibility of Chemical Reactions
If chemical bonds can be made, they can be broken, and vice versa.
Reversibility is indicated by a double arrow.
When the arrows differ in length, the longer arrow indicates the major direction in which the reaction proceeds:
A + B ⥂ AB
When the arrows are of equal length, as in neither the forward reaction nor the reverse reaction is dominant:
A + B ⇌ ABFor each molecule of product (AB) formed, one product molecule breaks down, releasing the reactants A and B.
Such a chemical reaction is said to be in a state of chemical equilibrium.
Once chemical equilibrium is reached, there is no further net change in the amounts of reactants and products unless more of either are added to the mix.
Product molecules are still formed and broken down, but the balance established when equilibrium was reached remains unchanged.
Chemical reactions that release energy will not go in the opposite direction unless energy is put back into the system.
Factors Influencing the Rate of Chemical Reactions
Temperature
Increasing the temperature of a substance increases the kinetic energy of its particles and the force of their collisions.
Concentration
Chemical reactions progress most rapidly when the reacting particles are present in high numbers, because the chance of successful collisions is greater
As concentration declines, the reaction slows.
The equilibrium occurs unless additional reactants are added or products are removed from the reaction site.
Particle Size
Smaller particles move faster than larger ones and tend to collide more frequently and more forcefully.
The smaller the reacting particles, the faster a chemical reaction goes at a given temperature and concentration.
Catalysts
These are substances that increase the rate of chemical reactions without themselves becoming chemically changed or part of the product.
Many chemical reactions in nonliving systems can be speeded up simply by heating, but drastic increases in body temperature are life threatening because important biological molecules are destroyed.
Enzymes: Biological catalysts.
Inorganic Compounds
Biochemistry: The study of the chemical composition and reactions of living matter.
Wet chemistry.
Organic compounds: Substances that contain carbon.
Inorganic compounds: Any substance in which two or more chemical elements are combined.
Water
The most abundant and important inorganic compound in a living material.
Properties of Water
High heat capacity
It absorbs and releases large amounts of heat before changing appreciably in temperature itself.
It prevents sudden changes in temperature caused by external factors or internal factors that release heat rapidly.
High heat of vaporization
When water evaporates, or vaporizes, it changes from a liquid to a gas.
This transformation requires that large amounts of heat be absorbed to break the hydrogen bonds that hold water molecules together.
Beneficial for sweating.
Polar solvent properties
Water is an unparalleled solvent.
Because water molecules are polar, they orient themselves with their slightly negative ends toward the positive ends of the solutes, and vice versa, first attracting the solute molecules, and then surrounding them.
Hydration layers: Layers of water molecules which water forms around large charged molecules.
Water is the body’s major transport medium
Reactivity
Water is an important reactant in many chemical reactions.
Hydrolysis reactions: Involves adding water to one large molecule to break it into multiple smaller molecules.
Dehydration synthesis: A reaction when a water molecule is removed for every bond formed.
Cushioning
By forming a resilient cushion around certain body organs, water helps protect them from physical trauma.
Ex.: Cerebrospinal fluid.
Salt
An ionic compound containing cations other than H+ and anions other than the hydroxyl ion (OH-)
All ions are electrolytes substances that conduct an electrical current in solution.
Polyatomic ions: Groups of atoms that bear an overall charge.
Sodium chloride, calcium carbonate and potassium chloride — common salts found in the body.
Calcium phosphates — salts that make bones and teeth hard.
The electrolyte properties of sodium and potassium ions are essential for nerve impulse transmission and muscle contraction.
Ionic iron forms part of the hemoglobin molecules that transport oxygen within red blood cells, and zinc and copper ions are important to the activity of some enzymes.
Maintaining proper ionic balance in our body fluids is one of the most crucial homeostatic roles of the kidneys. When this balance is severely disturbed, virtually nothing in the body works. All the physiological activities listed above and thousands of others are disrupted and grind to a stop.
Acids and Bases
Acids
Substances that releases hydrogen ions (H+) in detectable amounts.
Also defined as proton donors.
When acids dissolve in water, they release hydrogen ions (protons) and anions.
Bases
Substances that take up hydrogen ions in detectable amounts.
Also defined as proton acceptors.
Bicarbonate ion — an important base in the body, is particularly abundant in blood.
Ammonia — a common waste product of protein breakdown in the body, is also a base.
pH: Acid-Base Concentration
The more hydrogen ions in a solution, the more acidic the solution is.
The greater the concentration of hydroxyl ions, the more basic, or alkaline, the solution becomes.
pH units: Concentration units that measures relative concentration of hydrogen ions in various body fluids.
Sören Sörensen: He devised the pH scale in 1909.
The pH scale runs from 0 to 14 and is logarithmic.
Each successive change of one pH unit represents a tenfold change in hydrogen ion concentration.
The pH of a solution is thus defined as the negative logarithm of the hydrogen ion concentration in moles per liter.
Neutral — pH of 7.
Acidic — pH below 7.
Basic — pH above 7.
Neutralization reaction
A reaction when an acid and a base react to form water and a salt and involves the combination of H+ ions and OH- ions to generate water.
When acids and bases are mixed, they react with each other in displacement reactions to form water and a salt.
Buffers
Homeostasis of acid-base balance is carefully regulated by the kidneys and lungs and by chemical systems.
These resist abrupt and large swings in the pH of body fluids by releasing hydrogen ions when the pH begins to rise and by binding hydrogen ions when the pH drops.
Strong acids: Acids that dissociate completely and irreversibly in water.
hydrochloric acid and sulfuric acid
Weak acids: Acids that do not dissociate completely.
carbonic acid and acetic acid.
Strong bases: Bases that dissociate easily in water and quickly tie up H+.
Weak bases: Bases that do not completely dissociate into their constituent ions when dissolved in solutions.
Organic Compounds
Organic molecules are very large molecules, but their interactions with other molecules typically involve only small, reactive parts of their structure called functional groups.
Polymers: Chainlike molecules made of many similar or repeating units which are joined together by dehydration synthesis.
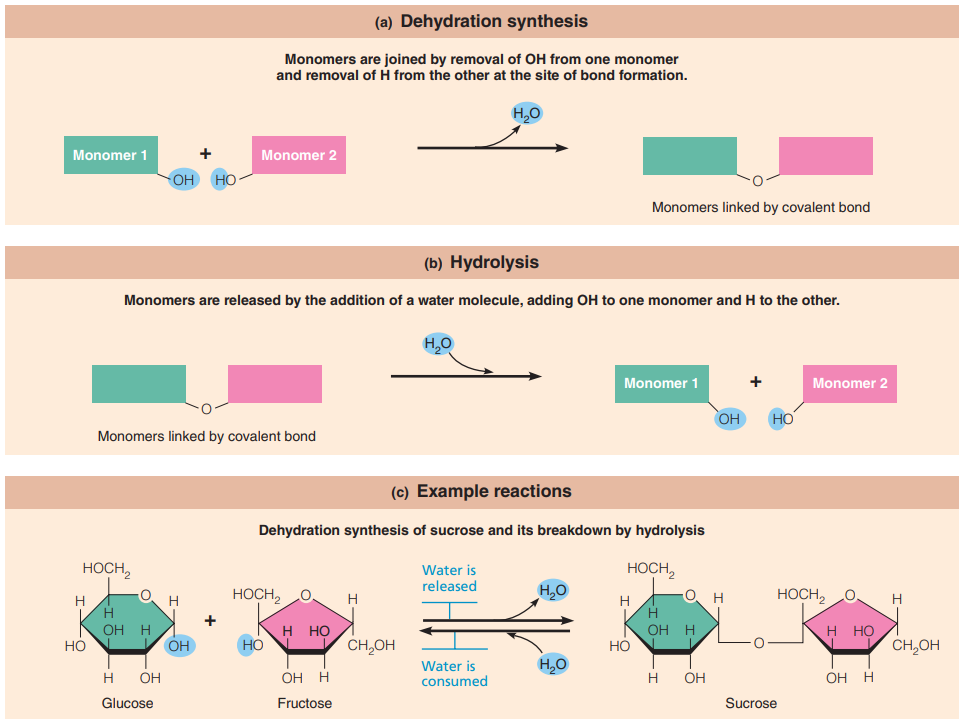
Carbohydrates
Carbohydrates: A group of molecules that includes sugars and starches, represent 1–2% of cell mass.
Its major function in the body is to provide a ready, easily used source of cellular fuel.
Monosaccharides: Simple sugars.
Single-chain or single-ring structures containing from three to seven carbon atoms.
Formula:
(CH2O)n— where n is the number of carbons in the sugar.These are named generically according to the number of carbon atoms they contain.
Deoxyribose: A pentose, — part of DNA.
Glucose: A hexose, — a blood sugar.
Galactose and fructose — isomers of glucose.
Isomers: Each of two or more compounds with the same formula but a different arrangement of atoms in the molecule and different properties.
Disaccharides: Double sugar.
Formed when two monosaccharides are joined by dehydration synthesis.
Sucrose: Cane or table sugar.
Lactose: Found in milk.
Maltose: Malt sugar.
Disaccharides are too large to pass through cell membranes, so they must be digested to their simple sugar units to be absorbed from the digestive tract into the blood — a hydrolysis.
Polysaccharides: Polymers of simple sugars linked together by dehydration synthesis.
They lack the sweetness of the simple and double sugars.
Starch: The storage carbohydrate formed by plants.
When we eat starchy foods, the starch must be digested for its glucose units to be absorbed.
We are unable to digest cellulose, another polysaccharide found in all plant products.
Cellulose: An insoluble substance which is the main constituent of plant cell walls and of vegetable fibers.
Glycogen: The storage carbohydrate of animal tissues, is stored primarily in skeletal muscle and liver cells.
When blood sugar levels drop sharply, liver cells break down glycogen and release its glucose units to the blood.
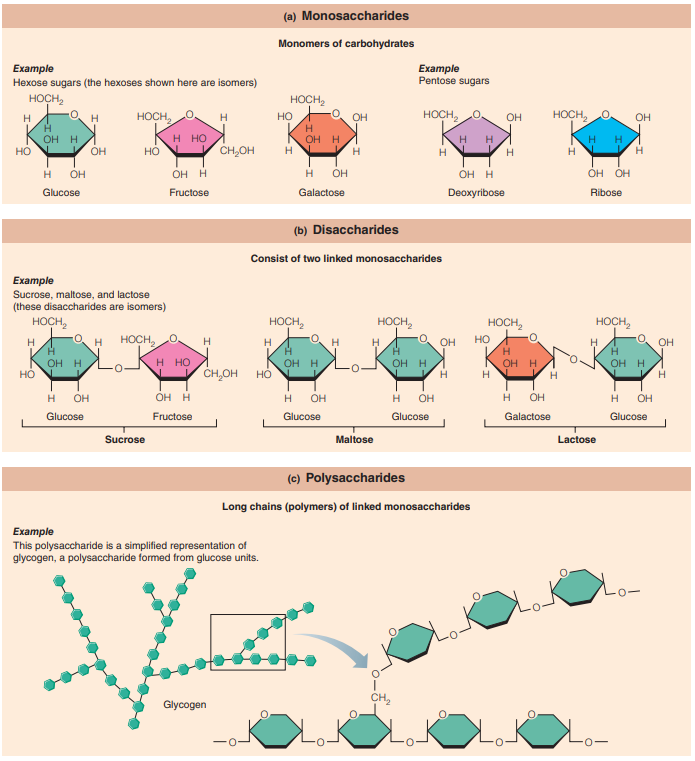
Lipids
Lipids: These are insoluble in water but dissolve readily in other lipids and in organic solvents such as alcohol and ether.
Contain carbon, hydrogen, and oxygen, but the proportion of oxygen in lipids is much lower.
Triglycerides: Neutral fats.
Commonly known as fats when solid or oils when liquid.
Composed of:
Fatty acids: Linear chains of carbon and hydrogen atoms with an organic acid group at one end.
Glycerol: A modified simple sugar (a sugar alcohol).
Fat deposits (in subcutaneous tissue and around organs) protect and insulate body organs, and are the major source of stored energy in the body.
Saturated fats: Fatty acid chains with only single covalent bonds between carbon atoms.
Unsaturated fats: Fatty acids that contain one bonds.
Polyunsaturated fats: Fatty acids that contain more double bonds between carbon atoms.
Triglycerides with short fatty acid chains or unsaturated fatty acids are oils.
Longer fatty acid chains and more saturated fatty acids are common in animal fats, which are solid at room temperature.
Trans fats: Oils that have been solidified by addition of H atoms at sites of carbon double bonds.
Omega-3 fatty acids: Found naturally in cold-water fish, appear to decrease the risk of heart disease and some inflammatory diseases.
Phospholipids: Modified triglycerides.
Diglycerides with a phosphorus-containing group and two, rather than three, fatty acid chains
Chief components of cell membranes.
Participate in the transport of lipids in plasma.
Prevalent in nervous tissue.
Although the hydrocarbon portion (the “tail”) of the molecule is nonpolar and interacts only with nonpolar molecules, the phosphorus-containing part (the “head”) is polar and attracts other polar or charged particles, such as water or ions.
Steroids: Flat molecules made of four interlocking hydrocarbon rings.
Fat soluble and contain little oxygen.
Cholesterol — most important molecule in our steroid chemistry.
Found in cell membranes and is the raw material for synthesis of vitamin D, steroid hormones, and bile salts.
Bile salts — These breakdown products of cholesterol are released by the liver into the digestive tract, where they aid fat digestion and absorption.
Vitamin D — A fat-soluble vitamin produced in the skin on exposure to UV radiation.
Necessary for normal bone growth and function.
Sex hormones — Estrogen and progesterone (female hormones) and testosterone (a male hormone) are produced in the gonads.
Necessary for normal reproductive function.
Adrenocortical hormones —
Cortisol — a glucocorticoid, is a metabolic hormone necessary for maintaining normal blood glucose levels.
Aldosterone — helps to regulate salt and water balance of the body by targeting the kidneys.
Fat-soluble vitamins
Vitamin A — Ingested in orange-pigmented vegetables and fruits.
Converted in the retina to retinal, a part of the photoreceptor pigment involved in vision.
Vitamin E — Ingested in plant products such as wheat germ and green leafy vegetables.
Claims have been made (but not proved in humans) that it promotes wound healing, contributes to fertility, and may help to neutralize highly reactive particles called free radicals believed to be involved in triggering some types of cancer.
Vitamin K — Made available to humans largely by the action of intestinal bacteria.
Also prevalent in a wide variety of foods.
Necessary for proper clotting of blood.
Eicosanoids: Group of molecules derived from fatty acids found in all cell membranes.
The potent prostaglandins have diverse effects, including stimulation of uterine contractions, regulation of blood pressure, control of gastrointestinal tract motility, and secretory activity.
Both prostaglandins and leukotrienes are involved in inflammation.
Thromboxanes are powerful vasoconstrictors.
Lipoproteins: Lipoid and protein-based substances that transport fatty acids and cholesterol in the bloodstream.
Major varieties are high-density lipoproteins (HDLs) and low-density lipoproteins (LDLs).
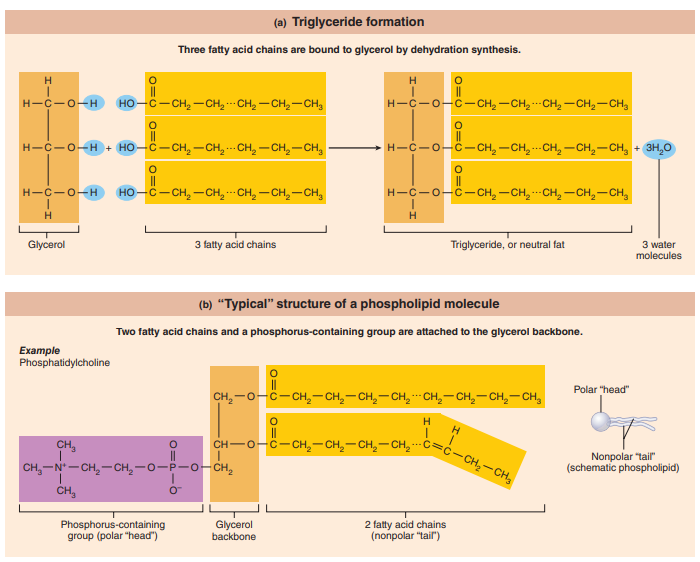
Proteins
Protein: Composes 10–30% of cell mass and is the basic structural material of the body.
Includes enzymes, hemoglobin of the blood, and contractile proteins of muscle, have the most varied functions of any molecules in the body.
Amino acids: Building blocks of proteins.
Two functional groups:
Amine group
Organic acid group
Peptide Bond: The resulting bond produces a characteristic arrangement of linked atoms.
Macromolecules: Large, complex molecules containing from 100 to over 10,000 amino acids.
Fibrous proteins: Extended and strandlike proteins.
Structural proteins.
Insoluble in water, and very stable—qualities ideal for providing mechanical support and tensile strength to the body’s tissues.
Collagen: A composite of the helical tropocollagen molecules packed together side by side to form a strong ropelike structure.
Globular proteins: Compact, spherical proteins that have at least tertiary structure.
Functional proteins.
Water-soluble, chemically active molecules, and they play crucial roles in virtually all biological processes.
Protein Denaturation: Occurs when hydrogen bonds begin to break when the pH drops or the temperature rises above normal (physiological) levels, causing proteins to unfold and lose their specific three-dimensional shape.
The disruption is reversible in most cases, and the “scrambled” protein regains its native structure when desirable conditions are restored.
If the temperature or pH change is so extreme that protein structure is damaged beyond repair, the protein is irreversibly denatured.
Active sites: Regions that fit and interact chemically with other molecules of complementary shape and charge.
Molecular Chaperones: Helps proteins to achieve their functional threedimensional structure.
Can associate with a broad range of “client” proteins, allowing them to perform a dizzying array of jobs
Prevent accidental, premature, or incorrect folding of polypeptide chains or their association with other polypeptides
Aid the desired folding and association process
Help to translocate proteins and certain metal ions (copper, iron, zinc) across cell membranes
Promote the breakdown of damaged or denatured proteins
Interact with immune cells to trigger the immune response to diseased cells in the body
Heat shock proteins: The first proteins discovered; they seemed to protect cells from the destructive effects of heat.
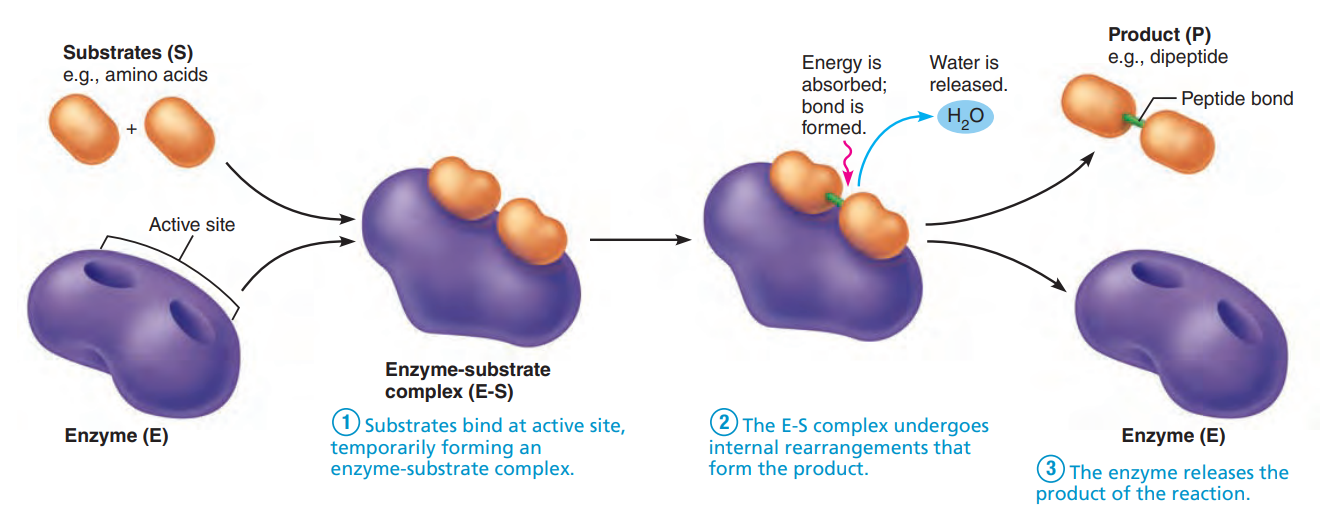
Enzymes: Globular proteins that act as biological catalysts.
Holoenzyme: A biochemically active compound formed by the combination of an enzyme with a coenzyme.
Apoenzyme: A protein that forms an active enzyme system by combination with a coenzyme and determines the specificity of this system for a substrate.
Cofactor: a non-protein molecule that supports a biochemical reaction.
Coenzyme: a nonprotein compound that is necessary for the functioning of an enzyme.
Substrate: The substance on which an enzyme acts.
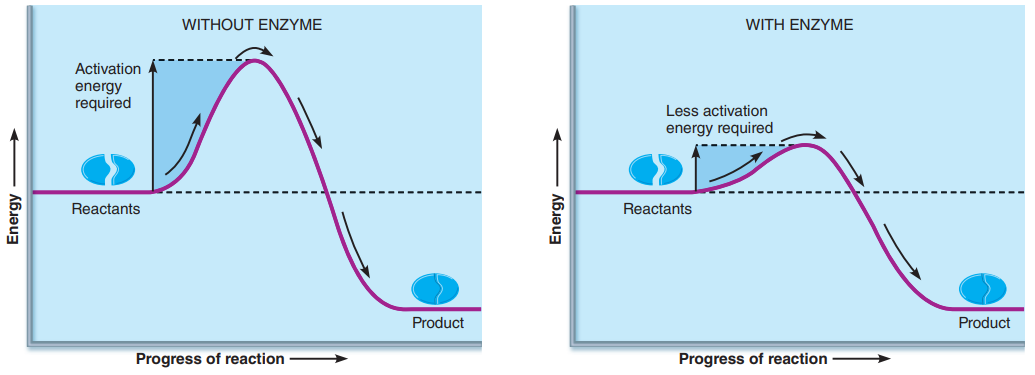
Activation energy: The amount of energy needed to break the bonds of the reactants so that they can rearrange themselves and become the product.
needed regardless of whether the overall reaction is ultimately energy absorbing or energy releasing.
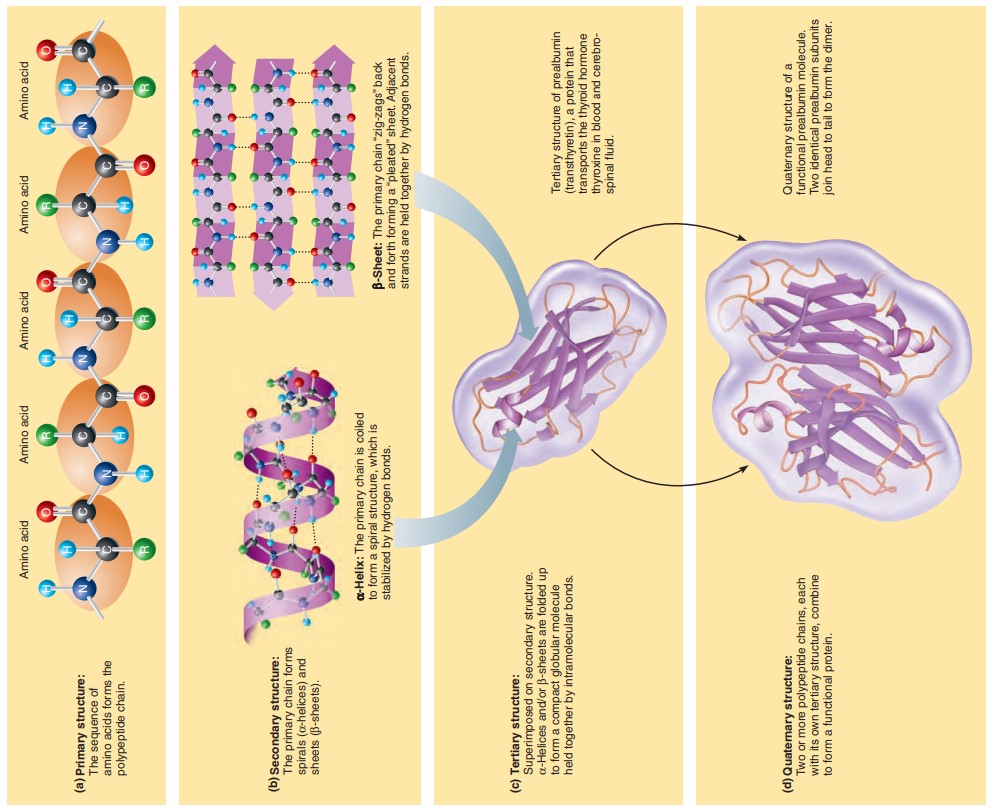
Structural Levels of Proteins
Primary structure of a protein — the linear sequence of amino acids composing the polypeptide chain.
Secondary structure of a protein — the primary chain twist or bend upon themselves to form this.
Alpha (α)-helix — Formed by coiling of the primary chain and is stabilized by hydrogen bonds formed between NH and CO groups in amino acids in the primary chain which are approximately four amino acids apart.
Beta (β)-pleated sheet — The primary polypeptide chains do not coil, but are linked side by side by hydrogen bonds to form a pleated, ribbonlike structure that resembles an accordion’s bellow.
Tertiary structure of a protein — Achieved when α-helical or β-pleated regions of the polypeptide chain fold upon one another to produce a compact ball-like, or globular, molecule.
Quaternary structure of a protein — When two or more polypeptide chains aggregate in a regular manner to form a complex protein.
Prealbumin: A protein that transports thyroid hormone in the blood.
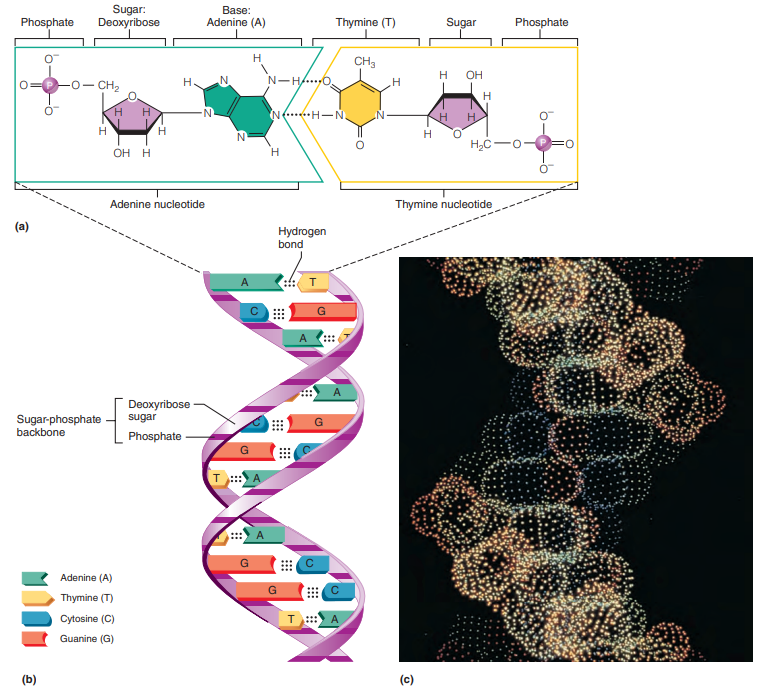
Nucleic Acids (DNA and RNA)
Nucleic acid: The largest molecules in the body.
Composed of carbon, oxygen, hydrogen, nitrogen, and phosphorus
Nucleotides: The structural units of nucleic acids
Three components — nitrogen-containing base, a pentose sugar, and a phosphate group
Five major varieties of nitrogen-containing bases — adenine, guanine, cytosine, thymine and uracil
The stepwise synthesis of a nucleotide involves the attachment of a base to the pentose sugar to form first a nucleoside, named for the nitrogenous base it contains.
The nucleotide is formed when a phosphate group is bonded to the sugar of the nucleoside.
DNA
Found in the nucleus of the cell, where it constitutes the genes,
A a long, double-stranded polymer — a double chain of nucleotides.
It replicates before a cell divides, ensuring that the genetic information in the descendant cells is identical.
It provides the basic instructions for building every protein in the body.
Alternating sugar and phosphate components of each chain form the backbones or “uprights” of the “ladder,” and the joined bases form the “rungs.”
Double helix: Spiral staircase–like structure of the DNA.
Complementary bases: A always bonds to T, and G always bonds to C.
DNA fingerprinting: It can help solve forensic mysteries, identify badly burned or mangled bodies at a disaster scene, and establish or disprove paternity.
It analyzes tiny samples of DNA taken from blood, semen, or other body tissues and shows the results as a “genetic barcode” that distinguishes each of us from all others.
RNA
Located chiefly outside the nucleus and can be considered a “molecular slave” of DNA.
Carries out the orders for protein synthesis issued by DNA.
Single strands of nucleotides.
Three major varieties — messenger RNA, ribosomal RNA, and transfer RNA.
MicroRNAs: Small RNA molecules; appear to control genetic expression by shutting down genes or altering their expression.
Comparison of DNA and RNA
CHARACTERISTIC | DNA | RNA |
|---|---|---|
Major cellular site | Nucleus | Cytoplasm |
Major functions | Is the genetic material; directs protein synthesis; replicates itself before cell division | Carries out the genetic instructions for protein synthesis |
Sugar | Deoxyribose | Ribose |
Bases | Adenine, guanine, cytosine, thymine | Adenine, guanine, cytosine, uracil |
Structure | Double strand coiled into a double helix | Single strand, straight or folded |
Adenosine Triphosphate (ATP)
Glucose: The most important cellular fuel, but none of the chemical energy contained in its bonds is used directly to power cellular work.
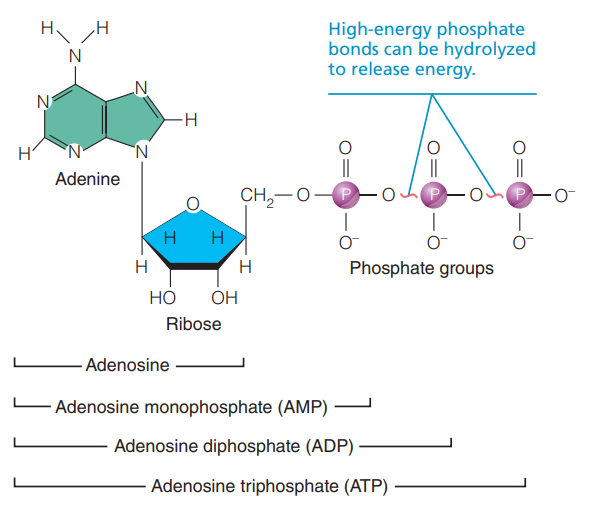
Adenosine Triphosphate (ATP): The primary energy-transferring molecule in cells and it provides a form of energy that is immediately usable by all body cells.
An adenine-containing RNA nucleotide to which two additional phosphate groups have been added. (Structure)
The triphosphate tail of ATP can be compared to a tightly coiled spring ready to uncoil with tremendous energy when the catch is released. (Chemical)
A very unstable energy-storing molecule because its three negatively charged phosphate groups are closely packed and repel each other.
When its terminal high-energy phosphate bonds are broken (hydrolyzed), the chemical “spring” relaxes and the molecule as a whole becomes more stable.
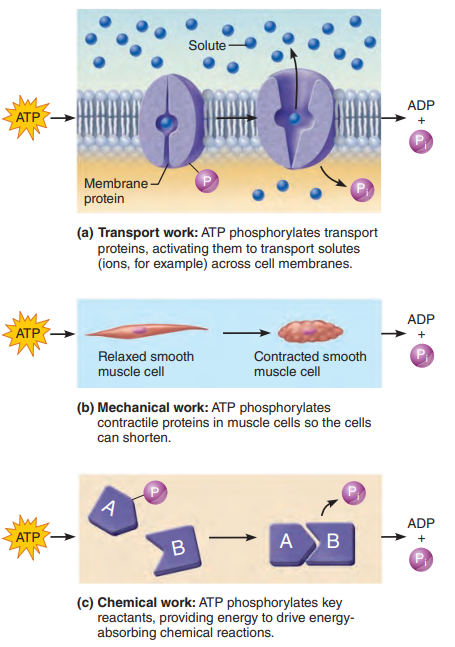
Without ATP, molecules cannot be made or degraded, cells cannot transport substances across their membrane boundaries, muscles cannot shorten to tug on other structures, and life processes cease
Adenosine Diphosphate: A compound consisting of an adenosine molecule bonded to three phosphate groups, present in all living tissue.
2: Chemistry Comes Alive
Matter and Energy
Matter
Matter: It is anything that occupies space and has mass.
The mass of an object is equal to the actual amount of matter in the object, and it remains constant wherever the object is.
Weight varies with gravity.
Solid: Has a definite shape and volume.
Liquid: Has a definite volume, but they conform to the shape of its container.
Gas: Has neither a definite shape nor a definite volume.
Energy
Energy: The capacity to do work, or to put matter into motion.
The greater the work done, the more energy is used doing it.
Kinetic energy: Energy in motion/action.
Potential energy: Stored energy, an inactive energy that has the capability to do work, but is not presently doing so.
Matter is the substance and energy is the mover of the substance.
Forms of Energy
Chemical energy: It is the form stored in the bonds of chemical substances.
Electrical energy: Energy that results from the movement of charged particles.
Mechanical energy: Energy directly involved in moving matter.
Radiant energy: Also called electromagnetic energy — Energy that travels in waves.
Composition of Matter: Atoms and Elements
Elements: Unique substances that cannot be broken down into simpler substances by ordinary chemical methods.
There are 118 elements that are currently recognized.
92 occur in nature and the rest are artificially made.
Periodic Table: A table that provides a more complete listing of the known elements and helps to explain the properties of each element that make it react as it does with other elements.
Atoms: Building blocks or, more or less identical particles of which an element is composed of.
The smallest atoms are less than 0.1 nanometer (nm) in diameter, and the largest are only about five times as large.
Physical properties of atoms: Those we can detect with our senses or measure.
Chemical properties of atoms: Pertain to the way atoms interact with other atoms (bonding behavior) and account for the facts that iron rusts, animals can digest their food, and so on.
Atomic Symbol: A one- or two-letter chemical shorthand used to designate each element, usually the first letter(s) of the element’s name.
Atomic Structure
An atom’s subatomic particles differ in mass, electrical charge, and position in the atom.
An atom has a central nucleus containing protons and neutrons tightly bound together.
Proton: A subatomic particle that bears a positive electrical charge.
Neutron: A subatomic particle that is neutral.
Electrons: A subatomic particle that bears a negative charge equal in strength to the positive charge of the proton.
Planetary Model: A simplified model of atomic structure.
Orbitals: Regions around the nucleus in which a given electron or electron pair is likely to be found most of the time.
Orbital Model: A more modern model of atomic structure, which is more useful for predicting the chemical behavior of atoms.


Common Elements Composing the Human Body
Element | % Body Mass | Function |
|---|---|---|
Oxygen (O) | 65.0 % | Needed for the production of ATP |
Carbon (C) | 18.5 % | A primary component of all organic molecules. |
Hydrogen (H) | 9.5 % | As an ion, it influences the pH of body fluids. |
Nitrogen (N) | 3.2 % | A component of proteins and nucleic acids. |
Calcium (Ca) | 1.5 % | Its ion form is required for muscle contraction, conduction of nerve impulses, and blood clotting. |
Phosphorus (P) | 1.0 % | Part of calcium phosphate salts in bones and teeth. |
Potassium (K) | 0.4 % | Necessary for conduction of nerve impulses and muscle contraction. |
Sulfur (S) | 0.3 % | Component of proteins, particularly muscle proteins |
Sodium (Na) | 0.2 % | Important for water balance, conduction of nerve impulses, and muscle contraction. |
Chlorine (Cl) | 0.2 % | Its ion is the most abundant negative ion (anion) in extracellular fluids. |
Magnesium (Mg) | 0.1 % | Also an important cofactor in a number of metabolic reactions. |
Iodine (I) | 0.1 % | Needed to make functional thyroid hormones. |
Iron (Fe) | 0.1 % | Component of hemoglobin and some enzymes. |
Identifying Elements
The atomic number of any atom is equal to the number of protons in its nucleus and is written as a subscript to the left of its atomic symbol.
The number of protons is always equal to the number of electrons in an atom, so the atomic number indirectly tells us the number of electrons in the atom as well.
The mass number of an atom is the sum of the masses of its protons and neutrons.
It is usually indicated by a superscript to the left of the atomic symbol.
Isotopes: A structural variation that all known elements have — it has the same number of protons, but differ in the number of neutrons they contain.
Atomic weight: An average of the relative weights (mass numbers) of all the isotopes of an element, taking into account their relative abundance in nature.
As a rule, the atomic weight of an element is approximately equal to the mass number of its most abundant isotope.
Radioactivity: The process of atomic decay.
Radioisotopes: Isotopes that exhibit the behaviour of radioactivity.
Half-Life: The time required for a radioisotope to lose one-half of its activity.
How Matter Is Combined: Molecules and Mixtures
Molecules and Compounds
Molecule: A combination of two or more atoms held together by chemical bonds.
If two or more atoms of the same element combine, the resulting substance is called a molecule of that element.
When two or more different kinds of atoms bind, they form molecules of a compound.
Mixture
Mixtures: These are substances composed of two or more components physically intermixed.
Solutions: These are homogeneous mixtures of components that may be gases, liquids, or solids.
Solvent: Substance present in greatest amounts.
Solute: Substance present in smaller amounts.
Water: The body’s chief solvent.
Most solutions in the body are true solutions containing gases, liquids, or solids dissolved in water. These solutions are usually transparent.
Solutions used in a college laboratory or a hospital are often described in terms of the percent of the solute in the total solution.
Milligrams per deciliter (mg/dl) is another common concentration measurement.
Molarity: A way to express the concentration of a solution — or moles per liter. A more complicated method but much more useful.
A mole of any element or compound is equal to its atomic weight or molecular weight weighed out in grams.

Avogadro’s number: One mole of any substance always contains exactly the same number of solute particles, that is, 6.02 x 10^23.
Colloid: Also called emulsion, — are heterogeneous mixtures, which means that their composition is dissimilar in different areas of the mixture.
It often appear translucent or milky and although the solute particles are larger than those in true solutions, they still do not settle out.
Sol-gel transformation: A unique property of colloid that change reversibly from a fluid (sol) state to a more solid (gel) state.
Cytosol: The semifluid material in living cells, is also a colloid, largely because of its dispersed proteins.
Suspensions: These are heterogeneous mixtures with large, often visible solutes that tend to settle out.

Chemical Bonds
Electrons forming the electron cloud around the nucleus of an atom occupy regions of space called electron shells that consecutively surround the atomic nucleus.
The atoms known so far can have electrons in seven shells, but the actual number of electron shells occupied in a given atom depends on the number of electrons that atom has.
The attraction between the positively charged nucleus and negatively charged electrons is greatest when electrons are closest to the nucleus and falls off with increasing distance.
This statement explains why electrons farthest from the nucleus
have the greatest potential energy, and
are most likely to interact chemically with other atoms.
When we consider bonding behavior, the only electrons that are important are those in the atom’s outermost energy level. Inner electrons usually do not take part in bonding because they are more tightly held by the atomic nucleus.
When the outermost energy level of an atom is filled to capacity or contains eight electrons, the atom is stable.
Such atoms are chemically inert, that is, unreactive.
A group of elements called the noble gases, which include helium and neon, typify this condition.
Atoms in which the outermost energy level contains fewer than eight electrons tend to gain, lose, or share electrons with other atoms to achieve stability.
The number of electrons that can participate in bonding is still limited to a total of eight.
Valence Shell: It indicates an atom’s outermost energy level or that portion of it containing the electrons that are chemically reactive.
The key to chemical reactivity is the octet rule or rule of eights.

Types of Chemical Bonds
Ionic Bond
Ionic Bond: A chemical bond between atoms formed by the transfer of one or more electrons from one atom to the other.
Ions: An atom or molecule with a net electric charge due to the loss or gain of one or more electrons.
Electron acceptor: The atom that gains one or more electrons.
Anion: A net negative charge.
Electron donor: The atom that loses electrons.
Cation: A net positive charge.
Both anions and cations are formed whenever electron transfer between atoms occurs. Because opposite charges attract, these ions tend to stay close together, resulting in an ionic bond.
Ionic bonds are commonly formed between atoms with one or two valence shell electrons and atoms with seven valence shell electrons .
Crystals: Large arrays of cations and anions held together by ionic bonds.


Covalent Bond
Covalent Bond: Electron sharing produces molecules in which the shared electrons occupy a single orbital common to both atoms.
Hydrogen with its single electron can fill its only shell (shell 1) by sharing a pair of electrons with another atom.
When it shares with another hydrogen atom, a molecule of hydrogen gas is formed.
The shared electron pair orbits around the molecule as a whole, satisfying the stability needs of each atom.
When two atoms share one pair of electrons, a single covalent bond is formed (indicated by a single line connecting the atoms, such as H¬H).
Nonpolar molecules: The molecules formed are electrically balanced. There has no separation of charge, so no positive or negative poles are formed.
Polar molecule: A molecule containing polar bonds where the sum of all the bond's dipole moments is not zero.
Electronegativity: The tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond.
Electropositivity: A measure of an element's ability to donate electrons, and therefore form positive ions; thus, it is antipode to electronegativity.
Dipole: Any molecule or radical that has delocalised positive and negative charges


Hydrogen Bond
Hydrogen bonds: It is formed when a hydrogen atom, already covalently linked to one electronegative atom (usually nitrogen or oxygen), is attracted by another electron-hungry atom, so that a “bridge” forms between them.
Hydrogen bonding is common between dipoles such as water molecules, because the slightly negative oxygen atoms of one molecule attract the slightly positive hydrogen atoms of other molecules.
Hydrogen bonding is responsible for the tendency of water molecules to cling together and form films, referred to as surface tension.
Although hydrogen bonds are too weak to bind atoms together to form molecules, they are important intramolecular bonds (literally, bonds within molecules), which hold different parts of a single large molecule in a specific three-dimensional shape.

Chemical Reactions
Chemical Reaction: It occurs whenever chemical bonds are formed, rearranged, or broken.
Chemical Equations
We can write chemical reactions in symbolic form as chemical equations. For example, we indicate the joining of two hydrogen atoms to form hydrogen gas as
H + H → H2 (hydrogen gas)and the combining of four hydrogen atoms and one carbon atom to form methane as
4H + C → CH4 (methane)
A number written as a subscript indicates that the atoms are joined by chemical bonds. But a number written as a prefix denotes the number of unjoined atoms or molecules.
CH4 reveals that four hydrogen atoms are bonded together with carbon to form the methane molecule, but 4H signifies four unjoined hydrogen atoms.
A chemical equation is like a sentence describing what happens in a reaction.
It contains the following information:
reactants, the number and kinds of reacting substances,
the chemical composition of the product(s)
in balanced equations, the relative proportion of each reactant and product.
Patterns of Chemical Reactions
Synthesis Reaction: Also known as a Combination reaction, it occurs when atoms or molecules combine to form a larger, more complex molecule.
A + B → AB
Bond formation.
Basis of constructive, or anabolic activities in body cells, such as joining small molecules called amino acids into large protein molecules.
Decomposition Reaction: This occurs when a molecule is broken down into smaller molecules or its constituent atoms.
AB → A + B
Bonds are broken.
Underlie all degradative, or catabolic, processes in body cells.
Exchange Reaction: Also known as the displacement reaction, it involve both synthesis and decomposition.
AB + C → AC + B and AB + CD → AD + CB
Bonds are both made and broken
Parts of the reactant molecules change partners, so to speak, producing different product molecules.
Occurs when ATP reacts with glucose and transfers its end phosphate group to glucose, forming glucose-phosphate. ATP becomes ADP, too.
Oxidation-Reduction Reaction: Also known as a redox reaction.
Decomposition reactions are the basis of all reactions in which food fuels are broken down for energy.
Here, electrons are exchanged between the reactants.
Electron donors — reactant losing the electrons; is said to be oxidised.
Electron acceptors — reactant taking up the transferred electrons; is said to be reduced.
This reaction also occurs when the ionic compound is formed.
Not all of these kinds of reactions involve the complete transfer of electrons — some change the pattern of electron sharing in covalent bonds.
Cellular Respiration: A series of chemical reactions that break down glucose to produce ATP, which may be used as energy to power many reactions throughout the body.
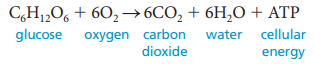

Energy Flow in Chemical Reactions
Exergonic reactions: Reactions that release energy.
Yield products with less energy than the initial reactants, along with energy that can be harvested for other uses.
Ex.: Catabolic and oxidative reactions
Endergonic reactions: Reactions that absorb energy.
Yield products that contain more potential energy in their chemical bonds than did the reactants
Ex.: Anabolic reactions
Reversibility of Chemical Reactions
If chemical bonds can be made, they can be broken, and vice versa.
Reversibility is indicated by a double arrow.
When the arrows differ in length, the longer arrow indicates the major direction in which the reaction proceeds:
A + B ⥂ AB
When the arrows are of equal length, as in neither the forward reaction nor the reverse reaction is dominant:
A + B ⇌ ABFor each molecule of product (AB) formed, one product molecule breaks down, releasing the reactants A and B.
Such a chemical reaction is said to be in a state of chemical equilibrium.
Once chemical equilibrium is reached, there is no further net change in the amounts of reactants and products unless more of either are added to the mix.
Product molecules are still formed and broken down, but the balance established when equilibrium was reached remains unchanged.
Chemical reactions that release energy will not go in the opposite direction unless energy is put back into the system.
Factors Influencing the Rate of Chemical Reactions
Temperature
Increasing the temperature of a substance increases the kinetic energy of its particles and the force of their collisions.
Concentration
Chemical reactions progress most rapidly when the reacting particles are present in high numbers, because the chance of successful collisions is greater
As concentration declines, the reaction slows.
The equilibrium occurs unless additional reactants are added or products are removed from the reaction site.
Particle Size
Smaller particles move faster than larger ones and tend to collide more frequently and more forcefully.
The smaller the reacting particles, the faster a chemical reaction goes at a given temperature and concentration.
Catalysts
These are substances that increase the rate of chemical reactions without themselves becoming chemically changed or part of the product.
Many chemical reactions in nonliving systems can be speeded up simply by heating, but drastic increases in body temperature are life threatening because important biological molecules are destroyed.
Enzymes: Biological catalysts.
Inorganic Compounds
Biochemistry: The study of the chemical composition and reactions of living matter.
Wet chemistry.
Organic compounds: Substances that contain carbon.
Inorganic compounds: Any substance in which two or more chemical elements are combined.
Water
The most abundant and important inorganic compound in a living material.
Properties of Water
High heat capacity
It absorbs and releases large amounts of heat before changing appreciably in temperature itself.
It prevents sudden changes in temperature caused by external factors or internal factors that release heat rapidly.
High heat of vaporization
When water evaporates, or vaporizes, it changes from a liquid to a gas.
This transformation requires that large amounts of heat be absorbed to break the hydrogen bonds that hold water molecules together.
Beneficial for sweating.
Polar solvent properties
Water is an unparalleled solvent.
Because water molecules are polar, they orient themselves with their slightly negative ends toward the positive ends of the solutes, and vice versa, first attracting the solute molecules, and then surrounding them.
Hydration layers: Layers of water molecules which water forms around large charged molecules.
Water is the body’s major transport medium
Reactivity
Water is an important reactant in many chemical reactions.
Hydrolysis reactions: Involves adding water to one large molecule to break it into multiple smaller molecules.
Dehydration synthesis: A reaction when a water molecule is removed for every bond formed.
Cushioning
By forming a resilient cushion around certain body organs, water helps protect them from physical trauma.
Ex.: Cerebrospinal fluid.
Salt
An ionic compound containing cations other than H+ and anions other than the hydroxyl ion (OH-)
All ions are electrolytes substances that conduct an electrical current in solution.
Polyatomic ions: Groups of atoms that bear an overall charge.
Sodium chloride, calcium carbonate and potassium chloride — common salts found in the body.
Calcium phosphates — salts that make bones and teeth hard.
The electrolyte properties of sodium and potassium ions are essential for nerve impulse transmission and muscle contraction.
Ionic iron forms part of the hemoglobin molecules that transport oxygen within red blood cells, and zinc and copper ions are important to the activity of some enzymes.
Maintaining proper ionic balance in our body fluids is one of the most crucial homeostatic roles of the kidneys. When this balance is severely disturbed, virtually nothing in the body works. All the physiological activities listed above and thousands of others are disrupted and grind to a stop.
Acids and Bases
Acids
Substances that releases hydrogen ions (H+) in detectable amounts.
Also defined as proton donors.
When acids dissolve in water, they release hydrogen ions (protons) and anions.
Bases
Substances that take up hydrogen ions in detectable amounts.
Also defined as proton acceptors.
Bicarbonate ion — an important base in the body, is particularly abundant in blood.
Ammonia — a common waste product of protein breakdown in the body, is also a base.
pH: Acid-Base Concentration
The more hydrogen ions in a solution, the more acidic the solution is.
The greater the concentration of hydroxyl ions, the more basic, or alkaline, the solution becomes.
pH units: Concentration units that measures relative concentration of hydrogen ions in various body fluids.
Sören Sörensen: He devised the pH scale in 1909.
The pH scale runs from 0 to 14 and is logarithmic.
Each successive change of one pH unit represents a tenfold change in hydrogen ion concentration.
The pH of a solution is thus defined as the negative logarithm of the hydrogen ion concentration in moles per liter.
Neutral — pH of 7.
Acidic — pH below 7.
Basic — pH above 7.
Neutralization reaction
A reaction when an acid and a base react to form water and a salt and involves the combination of H+ ions and OH- ions to generate water.
When acids and bases are mixed, they react with each other in displacement reactions to form water and a salt.
Buffers
Homeostasis of acid-base balance is carefully regulated by the kidneys and lungs and by chemical systems.
These resist abrupt and large swings in the pH of body fluids by releasing hydrogen ions when the pH begins to rise and by binding hydrogen ions when the pH drops.
Strong acids: Acids that dissociate completely and irreversibly in water.
hydrochloric acid and sulfuric acid
Weak acids: Acids that do not dissociate completely.
carbonic acid and acetic acid.
Strong bases: Bases that dissociate easily in water and quickly tie up H+.
Weak bases: Bases that do not completely dissociate into their constituent ions when dissolved in solutions.
Organic Compounds
Organic molecules are very large molecules, but their interactions with other molecules typically involve only small, reactive parts of their structure called functional groups.
Polymers: Chainlike molecules made of many similar or repeating units which are joined together by dehydration synthesis.

Carbohydrates
Carbohydrates: A group of molecules that includes sugars and starches, represent 1–2% of cell mass.
Its major function in the body is to provide a ready, easily used source of cellular fuel.
Monosaccharides: Simple sugars.
Single-chain or single-ring structures containing from three to seven carbon atoms.
Formula:
(CH2O)n— where n is the number of carbons in the sugar.These are named generically according to the number of carbon atoms they contain.
Deoxyribose: A pentose, — part of DNA.
Glucose: A hexose, — a blood sugar.
Galactose and fructose — isomers of glucose.
Isomers: Each of two or more compounds with the same formula but a different arrangement of atoms in the molecule and different properties.
Disaccharides: Double sugar.
Formed when two monosaccharides are joined by dehydration synthesis.
Sucrose: Cane or table sugar.
Lactose: Found in milk.
Maltose: Malt sugar.
Disaccharides are too large to pass through cell membranes, so they must be digested to their simple sugar units to be absorbed from the digestive tract into the blood — a hydrolysis.
Polysaccharides: Polymers of simple sugars linked together by dehydration synthesis.
They lack the sweetness of the simple and double sugars.
Starch: The storage carbohydrate formed by plants.
When we eat starchy foods, the starch must be digested for its glucose units to be absorbed.
We are unable to digest cellulose, another polysaccharide found in all plant products.
Cellulose: An insoluble substance which is the main constituent of plant cell walls and of vegetable fibers.
Glycogen: The storage carbohydrate of animal tissues, is stored primarily in skeletal muscle and liver cells.
When blood sugar levels drop sharply, liver cells break down glycogen and release its glucose units to the blood.

Lipids
Lipids: These are insoluble in water but dissolve readily in other lipids and in organic solvents such as alcohol and ether.
Contain carbon, hydrogen, and oxygen, but the proportion of oxygen in lipids is much lower.
Triglycerides: Neutral fats.
Commonly known as fats when solid or oils when liquid.
Composed of:
Fatty acids: Linear chains of carbon and hydrogen atoms with an organic acid group at one end.
Glycerol: A modified simple sugar (a sugar alcohol).
Fat deposits (in subcutaneous tissue and around organs) protect and insulate body organs, and are the major source of stored energy in the body.
Saturated fats: Fatty acid chains with only single covalent bonds between carbon atoms.
Unsaturated fats: Fatty acids that contain one bonds.
Polyunsaturated fats: Fatty acids that contain more double bonds between carbon atoms.
Triglycerides with short fatty acid chains or unsaturated fatty acids are oils.
Longer fatty acid chains and more saturated fatty acids are common in animal fats, which are solid at room temperature.
Trans fats: Oils that have been solidified by addition of H atoms at sites of carbon double bonds.
Omega-3 fatty acids: Found naturally in cold-water fish, appear to decrease the risk of heart disease and some inflammatory diseases.
Phospholipids: Modified triglycerides.
Diglycerides with a phosphorus-containing group and two, rather than three, fatty acid chains
Chief components of cell membranes.
Participate in the transport of lipids in plasma.
Prevalent in nervous tissue.
Although the hydrocarbon portion (the “tail”) of the molecule is nonpolar and interacts only with nonpolar molecules, the phosphorus-containing part (the “head”) is polar and attracts other polar or charged particles, such as water or ions.
Steroids: Flat molecules made of four interlocking hydrocarbon rings.
Fat soluble and contain little oxygen.
Cholesterol — most important molecule in our steroid chemistry.
Found in cell membranes and is the raw material for synthesis of vitamin D, steroid hormones, and bile salts.
Bile salts — These breakdown products of cholesterol are released by the liver into the digestive tract, where they aid fat digestion and absorption.
Vitamin D — A fat-soluble vitamin produced in the skin on exposure to UV radiation.
Necessary for normal bone growth and function.
Sex hormones — Estrogen and progesterone (female hormones) and testosterone (a male hormone) are produced in the gonads.
Necessary for normal reproductive function.
Adrenocortical hormones —
Cortisol — a glucocorticoid, is a metabolic hormone necessary for maintaining normal blood glucose levels.
Aldosterone — helps to regulate salt and water balance of the body by targeting the kidneys.
Fat-soluble vitamins
Vitamin A — Ingested in orange-pigmented vegetables and fruits.
Converted in the retina to retinal, a part of the photoreceptor pigment involved in vision.
Vitamin E — Ingested in plant products such as wheat germ and green leafy vegetables.
Claims have been made (but not proved in humans) that it promotes wound healing, contributes to fertility, and may help to neutralize highly reactive particles called free radicals believed to be involved in triggering some types of cancer.
Vitamin K — Made available to humans largely by the action of intestinal bacteria.
Also prevalent in a wide variety of foods.
Necessary for proper clotting of blood.
Eicosanoids: Group of molecules derived from fatty acids found in all cell membranes.
The potent prostaglandins have diverse effects, including stimulation of uterine contractions, regulation of blood pressure, control of gastrointestinal tract motility, and secretory activity.
Both prostaglandins and leukotrienes are involved in inflammation.
Thromboxanes are powerful vasoconstrictors.
Lipoproteins: Lipoid and protein-based substances that transport fatty acids and cholesterol in the bloodstream.
Major varieties are high-density lipoproteins (HDLs) and low-density lipoproteins (LDLs).

Proteins
Protein: Composes 10–30% of cell mass and is the basic structural material of the body.
Includes enzymes, hemoglobin of the blood, and contractile proteins of muscle, have the most varied functions of any molecules in the body.
Amino acids: Building blocks of proteins.
Two functional groups:
Amine group
Organic acid group
Peptide Bond: The resulting bond produces a characteristic arrangement of linked atoms.
Macromolecules: Large, complex molecules containing from 100 to over 10,000 amino acids.
Fibrous proteins: Extended and strandlike proteins.
Structural proteins.
Insoluble in water, and very stable—qualities ideal for providing mechanical support and tensile strength to the body’s tissues.
Collagen: A composite of the helical tropocollagen molecules packed together side by side to form a strong ropelike structure.
Globular proteins: Compact, spherical proteins that have at least tertiary structure.
Functional proteins.
Water-soluble, chemically active molecules, and they play crucial roles in virtually all biological processes.
Protein Denaturation: Occurs when hydrogen bonds begin to break when the pH drops or the temperature rises above normal (physiological) levels, causing proteins to unfold and lose their specific three-dimensional shape.
The disruption is reversible in most cases, and the “scrambled” protein regains its native structure when desirable conditions are restored.
If the temperature or pH change is so extreme that protein structure is damaged beyond repair, the protein is irreversibly denatured.
Active sites: Regions that fit and interact chemically with other molecules of complementary shape and charge.
Molecular Chaperones: Helps proteins to achieve their functional threedimensional structure.
Can associate with a broad range of “client” proteins, allowing them to perform a dizzying array of jobs
Prevent accidental, premature, or incorrect folding of polypeptide chains or their association with other polypeptides
Aid the desired folding and association process
Help to translocate proteins and certain metal ions (copper, iron, zinc) across cell membranes
Promote the breakdown of damaged or denatured proteins
Interact with immune cells to trigger the immune response to diseased cells in the body
Heat shock proteins: The first proteins discovered; they seemed to protect cells from the destructive effects of heat.
Enzymes: Globular proteins that act as biological catalysts.
Holoenzyme: A biochemically active compound formed by the combination of an enzyme with a coenzyme.
Apoenzyme: A protein that forms an active enzyme system by combination with a coenzyme and determines the specificity of this system for a substrate.
Cofactor: a non-protein molecule that supports a biochemical reaction.
Coenzyme: a nonprotein compound that is necessary for the functioning of an enzyme.
Substrate: The substance on which an enzyme acts.
Activation energy: The amount of energy needed to break the bonds of the reactants so that they can rearrange themselves and become the product.
needed regardless of whether the overall reaction is ultimately energy absorbing or energy releasing.



Structural Levels of Proteins
Primary structure of a protein — the linear sequence of amino acids composing the polypeptide chain.
Secondary structure of a protein — the primary chain twist or bend upon themselves to form this.
Alpha (α)-helix — Formed by coiling of the primary chain and is stabilized by hydrogen bonds formed between NH and CO groups in amino acids in the primary chain which are approximately four amino acids apart.
Beta (β)-pleated sheet — The primary polypeptide chains do not coil, but are linked side by side by hydrogen bonds to form a pleated, ribbonlike structure that resembles an accordion’s bellow.
Tertiary structure of a protein — Achieved when α-helical or β-pleated regions of the polypeptide chain fold upon one another to produce a compact ball-like, or globular, molecule.
Quaternary structure of a protein — When two or more polypeptide chains aggregate in a regular manner to form a complex protein.
Prealbumin: A protein that transports thyroid hormone in the blood.

Nucleic Acids (DNA and RNA)
Nucleic acid: The largest molecules in the body.
Composed of carbon, oxygen, hydrogen, nitrogen, and phosphorus
Nucleotides: The structural units of nucleic acids
Three components — nitrogen-containing base, a pentose sugar, and a phosphate group
Five major varieties of nitrogen-containing bases — adenine, guanine, cytosine, thymine and uracil
The stepwise synthesis of a nucleotide involves the attachment of a base to the pentose sugar to form first a nucleoside, named for the nitrogenous base it contains.
The nucleotide is formed when a phosphate group is bonded to the sugar of the nucleoside.
DNA
Found in the nucleus of the cell, where it constitutes the genes,
A a long, double-stranded polymer — a double chain of nucleotides.
It replicates before a cell divides, ensuring that the genetic information in the descendant cells is identical.
It provides the basic instructions for building every protein in the body.
Alternating sugar and phosphate components of each chain form the backbones or “uprights” of the “ladder,” and the joined bases form the “rungs.”
Double helix: Spiral staircase–like structure of the DNA.
Complementary bases: A always bonds to T, and G always bonds to C.
DNA fingerprinting: It can help solve forensic mysteries, identify badly burned or mangled bodies at a disaster scene, and establish or disprove paternity.
It analyzes tiny samples of DNA taken from blood, semen, or other body tissues and shows the results as a “genetic barcode” that distinguishes each of us from all others.
RNA
Located chiefly outside the nucleus and can be considered a “molecular slave” of DNA.
Carries out the orders for protein synthesis issued by DNA.
Single strands of nucleotides.
Three major varieties — messenger RNA, ribosomal RNA, and transfer RNA.
MicroRNAs: Small RNA molecules; appear to control genetic expression by shutting down genes or altering their expression.
Comparison of DNA and RNA
CHARACTERISTIC | DNA | RNA |
|---|---|---|
Major cellular site | Nucleus | Cytoplasm |
Major functions | Is the genetic material; directs protein synthesis; replicates itself before cell division | Carries out the genetic instructions for protein synthesis |
Sugar | Deoxyribose | Ribose |
Bases | Adenine, guanine, cytosine, thymine | Adenine, guanine, cytosine, uracil |
Structure | Double strand coiled into a double helix | Single strand, straight or folded |

Adenosine Triphosphate (ATP)
Glucose: The most important cellular fuel, but none of the chemical energy contained in its bonds is used directly to power cellular work.
Adenosine Triphosphate (ATP): The primary energy-transferring molecule in cells and it provides a form of energy that is immediately usable by all body cells.
An adenine-containing RNA nucleotide to which two additional phosphate groups have been added. (Structure)
The triphosphate tail of ATP can be compared to a tightly coiled spring ready to uncoil with tremendous energy when the catch is released. (Chemical)
A very unstable energy-storing molecule because its three negatively charged phosphate groups are closely packed and repel each other.
When its terminal high-energy phosphate bonds are broken (hydrolyzed), the chemical “spring” relaxes and the molecule as a whole becomes more stable.
Without ATP, molecules cannot be made or degraded, cells cannot transport substances across their membrane boundaries, muscles cannot shorten to tug on other structures, and life processes cease
Adenosine Diphosphate: A compound consisting of an adenosine molecule bonded to three phosphate groups, present in all living tissue.


 Knowt
Knowt
