
Chapter 16- The Periodic Table
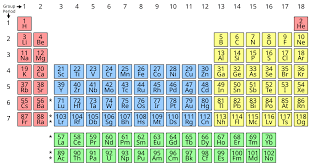
The periodic table is a list of elements arranged in order of increasing proton number.
It is divided into horizontal rows called periods and vertical columns called groups.
There are seven periods and eight groups. The eighth group is sometimes called the group O.
Between group II and III, there is a block of metals called transition metals.
GENERAL TRENDS OF A PERIODIC TABLE
Group--- vertical column
Period--- horizontal row
There is a decrease in metallic properties across a period
Same group--- same valency/number of valence electrons
Same period--- same number of shells
Left to right--- gradual change from metals to non-metals
Group I to Group III--- lose electrons to form cations
Group V to Group VII--- gain electrons to form anions
Group IV--- can either gain or lose electrons
Transition elements between Group II and III have variable valency
Group VIII/O--- are stable and do not react
Atomic size--- decreases across a period, increases down a group.
The group number determines the number of valance electrons (and thus, valency) of the element while the period determines the number of electron shells in an atom of that element.
ALKALI METALS- GROUP I
Properties:
Soft, can be easily cut
Low melting and boiling points
Low densities, are light
Good thermal and electrical conductors
Highly reactive
React with cold water to form alkali and hydrogen: 2Na + 2H20 2NaOH + H20
React with halogens to form halides: 2Na + Cl2 2NaCl
Form ionic compounds
Are powerful reducing agents
Trends:
Increases down the group: density, reactivity, softness
Decreases down the group: Melting and boiling points
HALOGENS- GROUP VII
Properties:
Coloured
Low melting and boiling points
Highly reactive
Exist in diatomic state
Undergo displacement reactions: 2NaBr + Cl2 2NaCl + Br2
Are powerful oxidizing agents
Trends:
Increases down the group: Melting and boiling points, density, colour (darker)
Decreases down the group: Reactivity, state (gas to solid)
NOBLE GASES- GROUP O
Properties:
Low melting and boiling points
Colourless
Insoluble in water
Unreactive, provide inert situations
Trends:
1) Increases down the group: Boiling point, mass
Uses:
Helium: for filling balloons and airships
Argon: for filling tungsten bulbs
Neon: used in lights and advertising signs
Xenon: used in vehicle headlamps
Argon: to provide inert atmosphere for processes like welding
Oxygen: welding, oxygen tents and tanks.
Chapter 16- The Periodic Table
The periodic table is a list of elements arranged in order of increasing proton number.
It is divided into horizontal rows called periods and vertical columns called groups.
There are seven periods and eight groups. The eighth group is sometimes called the group O.
Between group II and III, there is a block of metals called transition metals.
GENERAL TRENDS OF A PERIODIC TABLE
Group--- vertical column
Period--- horizontal row
There is a decrease in metallic properties across a period
Same group--- same valency/number of valence electrons
Same period--- same number of shells
Left to right--- gradual change from metals to non-metals
Group I to Group III--- lose electrons to form cations
Group V to Group VII--- gain electrons to form anions
Group IV--- can either gain or lose electrons
Transition elements between Group II and III have variable valency
Group VIII/O--- are stable and do not react
Atomic size--- decreases across a period, increases down a group.
The group number determines the number of valance electrons (and thus, valency) of the element while the period determines the number of electron shells in an atom of that element.
ALKALI METALS- GROUP I
Properties:
Soft, can be easily cut
Low melting and boiling points
Low densities, are light
Good thermal and electrical conductors
Highly reactive
React with cold water to form alkali and hydrogen: 2Na + 2H20 2NaOH + H20
React with halogens to form halides: 2Na + Cl2 2NaCl
Form ionic compounds
Are powerful reducing agents
Trends:
Increases down the group: density, reactivity, softness
Decreases down the group: Melting and boiling points
HALOGENS- GROUP VII
Properties:
Coloured
Low melting and boiling points
Highly reactive
Exist in diatomic state
Undergo displacement reactions: 2NaBr + Cl2 2NaCl + Br2
Are powerful oxidizing agents
Trends:
Increases down the group: Melting and boiling points, density, colour (darker)
Decreases down the group: Reactivity, state (gas to solid)
NOBLE GASES- GROUP O
Properties:
Low melting and boiling points
Colourless
Insoluble in water
Unreactive, provide inert situations
Trends:
1) Increases down the group: Boiling point, mass
Uses:
Helium: for filling balloons and airships
Argon: for filling tungsten bulbs
Neon: used in lights and advertising signs
Xenon: used in vehicle headlamps
Argon: to provide inert atmosphere for processes like welding
Oxygen: welding, oxygen tents and tanks.

 Knowt
Knowt
