
Chapter 13: Mutations and Genetic Testing
13.1 Gene Mutations
A gene is a sequence of DNA bases that codes for a cellular product, usually a protein.
Variations in genes are called alleles.
Alleles can code for observable traits like flower color or wing shape or for non-observable traits like susceptibility to medication.
New alleles are formed through mutations or changes in the DNA sequence.
Mutations can have negative consequences, like the alleles for cystic fibrosis.
Mutations can also create new gene products with positive functions for the organism.
Mutations play an important role in the process of evolution.
Causes of Gene Mutations
Gene mutations can be caused by errors in DNA replication, transposons, or environmental mutagens.
Mutations due to DNA replication errors are rare, occurring with a frequency of 1 in 100 million cell divisions on average in most eukaryotes.
DNA polymerase is the enzyme that carries out replication and proofreads the new strand against the old strand, detecting and correcting any mismatched pairs, which contributes to the low frequency of replication errors.
Mutagens are environmental influences that cause mutations, such as different forms of radiation and chemical mutagens, like pesticides and compounds in cigarette smoke.
If mutagens cause a mutation in an individual's gametes, their offspring may be affected. If the mutation occurs in the individual's body cells, cancer may result.
DNA repair enzymes constantly monitor for any irregularity and remedy the problem, contributing to the low overall rate of mutation.
Transposons are specific DNA sequences that can move within and between chromosomes, often disrupting genes and rendering them nonfunctional.
Transposons have been discovered in almost every group of organisms, including bacteria, plants, fruit flies, and humans.
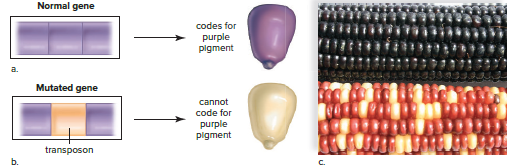
Types and Effects of Mutations
Effects of mutations:
Mutations can have a wide range of effects, from no effect to severe disease.
The severity of a mutation depends on the type of mutation and the gene involved.
Silent mutations are mutations that have no effect on the protein produced by the gene.
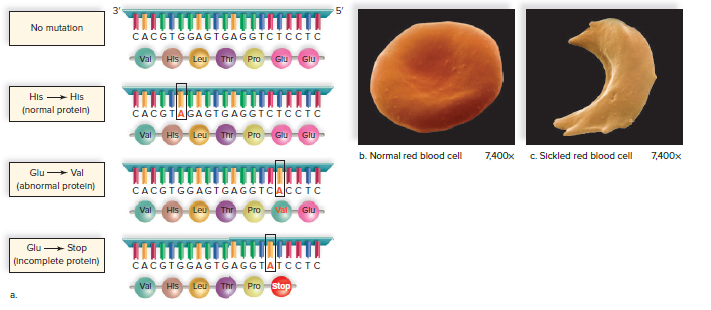
Point mutations are mutations that change a single base pair in the DNA.
Frameshift mutations are mutations that add or delete a base pair in the DNA.
Point mutations:
Point mutations can change the amino acid that is coded for by a gene.
Some point mutations are harmless, while others can cause disease.
Sickle cell anemia is a disease that is caused by a point mutation in the gene for hemoglobin.
Frameshift mutations:
Frameshift mutations can change the reading frame of the DNA.
This can cause the protein that is produced by the gene to be nonfunctional.
Fragile X syndrome is a disease that is caused by a frameshift mutation in the FMR1 gene.
13.2 Chromosomal Mutations
Mutations can cause changes in the number of chromosomes or the structure of individual chromosomes.
In humans, variations in chromosome number are typically caused by nondisjunction events during meiosis.
Changes in chromosome structure are more common and can be caused by environmental agents.
Syndromes resulting from changes in chromosome structure are due to the breakage of chromosomes and failure to reunite properly.
Chromosomal mutations can result in deletion, duplication, translocation, or inversion.
Chromosomal mutations can occur during meiosis and may result in a syndrome if the offspring inherits the abnormal chromosome.
Deletions and Duplications
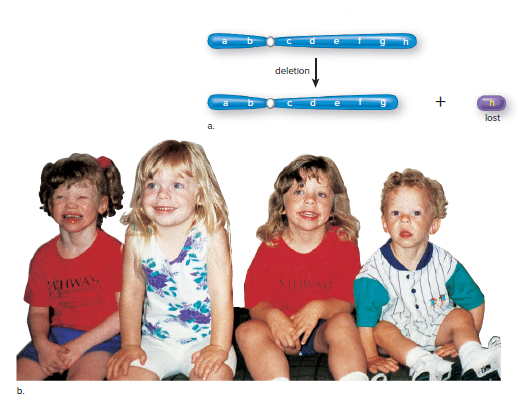
Deletion:
A single break causes the loss of an end piece.
Two simultaneous breaks lead to the loss of an internal segment.
Inheritance of a normal chromosome from one parent and a chromosome with deletion from another parent results in the loss of pair of alleles and can cause the syndrome.
Williams Syndrome:
Occurs when chromosome 7 loses a tiny endpiece.
Symptoms: turned-up nose, wide mouth, small chin, large ears.
Poor academic skills, excellent verbal and musical abilities.
The missing gene for elastin protein affects cardiovascular health and causes premature skin aging.
Friendly but needs ordered life due to loss of gene for a brain protein
Cri du chat Syndrome:
Occurs when chromosome 5 is missing the end piece.
Symptoms: small head, mental disabilities, facial abnormalities.
Abnormal development of the glottis and larynx results in cat-like cries.
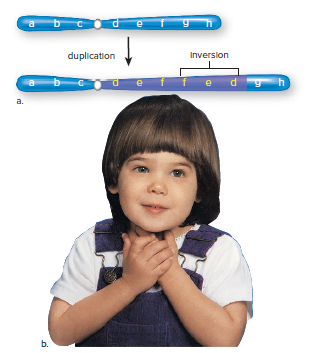
Duplication: chromosome segment is repeated, leading to more than two alleles for certain traits.
Inverted duplication: segment runs in the opposite direction from normal.
Occurs in chromosome 15.
Inv dup 15 syndrome: children have poor muscle tone, mental disabilities, seizures, curved spine, and autistic characteristics.
Autistic characteristics include poor speech, hand flapping, and lack of eye contact.
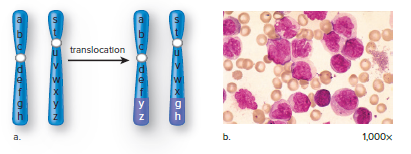
Translocation
A translocation is the movement of a segment from one chromosome to another, a nonhomologous chromosome, or the exchange of segments between nonhomologous chromosomes.
A person who has both of the involved chromosomes has the normal amount of genetic material and a normal phenotype unless the translocation breaks an allele into two pieces or fuses two genes together.
The person who inherits only one of the translocated chromosomes will have only one copy of certain alleles and three copies of other alleles.
A genetic counselor begins to suspect a translocation has occurred when spontaneous abortions are commonplace, and family members suffer from various syndromes.
In 5% of Down syndrome cases, a translocation that occurred in a previous generation between chromosomes 21 and 14 is the cause.
Some forms of cancer are also associated with translocations, such as chronic myeloid leukemia (CML) and Burkitt’s lymphoma.
Alagille syndrome is caused by a translocation between chromosomes 2 and 20, which often produces a deletion on chromosome 20.
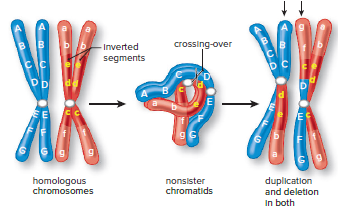
Inversion
An inversion occurs when a segment of a chromosome is turned 180° (Fig. 13.6).
The reversed sequence of alleles can lead to altered gene activity if it disrupts the control of gene expression.
Inversions usually do not cause problems, but they can lead to an increased occurrence of abnormal chromosomes during sexual reproduction.
Crossing over between an inverted chromosome and the noninverted homologue can lead to recombinant chromosomes that have both duplicated and deleted segments.
Alignment between the two homologs is only possible when the inverted chromosome forms a loop (Fig. 13.6).
13.3 Genetic Testing
Potential parents are becoming aware of the link between abnormal chromosomal inheritance or gene mutations and illnesses.
More couples are seeking genetic counseling to determine the risk of inherited disorders in their family.
Couples may seek counseling after experiencing several miscarriages, having relatives with a medical condition, or having a child with a genetic defect.
The counselor helps the couple understand the mode of inheritance, the medical consequences of a genetic disorder, and their options.
Abnormal chromosome numbers or structures can result in various human disorders.
If a pregnant woman is concerned about a chromosomal defect in her unborn child, the counselor may recommend karyotyping the fetus's chromosomes.
Analyzing the Chromosomes
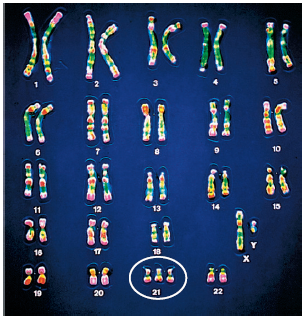
A karyotype is a visual display of pairs of chromosomes arranged by size, shape, and banding pattern.
Any cell in the body except red blood cells can be a source of chromosomes for karyotyping.
White blood cells separated from a blood sample are the easiest to use for karyotyping in adults.
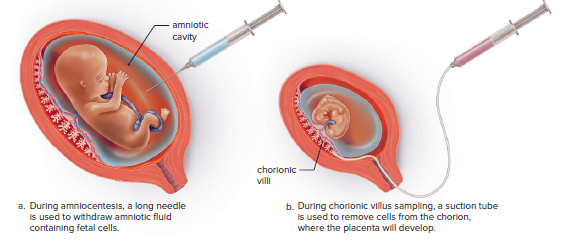
Cells can be obtained by either amniocentesis or chorionic villus sampling in fetuses.
The karyotype of a person with Down syndrome usually has three copies of chromosome number 21 instead of two.
Amniocentesis is a procedure for obtaining a sample of amniotic fluid from the uterus of a pregnant woman.
Chorionic villus sampling (CVS) is a procedure for obtaining chorionic villi cells in the region where the placenta will develop.
After a cell sample has been obtained, the cells are stimulated to divide in a culture medium.
Stains are applied to the slides, and the cells are photographed to create a traditional karyotype.
Following genetic testing, a genetic counselor can explain to prospective parents the chances that a child of theirs will have a disorder that runs in the family.
Testing for a Protein
Genetic mutations can cause disorders due to a lack of enzyme activity.
Methemoglobinemia is an example of such a disorder.
Diaphorase enzyme quantity in blood samples can determine if an individual is a homozygous normal carrier or has methemoglobinemia.
If parents are carriers, each child has a 25% chance of having methemoglobinemia.
Prospective parents may opt for embryo or egg testing based on this knowledge.
Testing DNA
Methods of analyzing DNA for specific mutations:
Testing for a genetic marker.
Using a DNA microarray.
Direct sequencing of an individual's DNA.
Genetic Markers
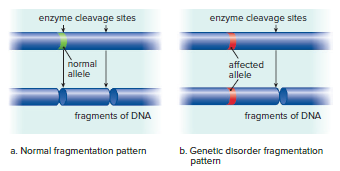
Testing for a genetic marker relies on a difference in DNA due to the presence of an abnormal allele.
Individuals with sickle-cell disease or Huntington's disease have an abnormality in a gene's base sequence, which serves as a genetic marker.
DNA sequencing can detect the presence of specific genetic markers.
Restriction enzymes can be used to cleave DNA at particular base sequences.
The fragments resulting from the use of a restriction enzyme may differ for people who are normal than for those who are heterozygous or homozygous for a mutation.
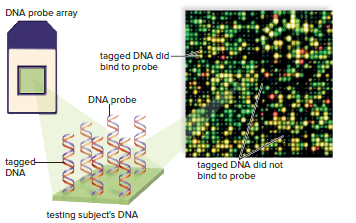
DNA Microarrays
New technologies have made DNA testing easy and inexpensive.
DNA microarray or gene chip is a small silicon chip containing many DNA samples.
Thousands of known disease-associated mutant alleles can be placed onto a DNA microarray.
Genomic DNA from the subject to be tested is labeled with a fluorescent dye and added to the microarray.
The spots on the microarray fluoresce if the DNA binds to the mutant alleles on the chip, indicating that the subject may have a particular disorder or is at risk of developing it later in life.
An individual’s complete genotype, including all the various mutations, is called a genetic profile.
With the help of a genetic counselor, individuals can be educated about their genetic profile.
A genetic profile can indicate an increased or decreased risk for a disorder.
Risk information can be used to design a program of medical surveillance and to foster a lifestyle aimed at reducing the risk.
For example, an individual with mutations common to people with colon cancer should have an annual colonoscopy to detect and remove any abnormal growths before they become invasive.
DNA Sequencing
Recent advances in DNA sequencing have made it more economically feasible to sequence an individual's genome to detect specific mutations associated with a disease.
The cost of sequencing an individual's genome has decreased from $100,000 to almost $1,000, ushering in an era of personal genomics or personalized medicine.
Personal genomics can be approached by sequencing the entire genome of an individual or targeting specific genes and looking for alleles that increase the risk associated with a specific disease.
Genome-wide association study has become more prevalent in personalized medicine due to the increase in large genomic population studies.
Pharmacogenomics is the selection of a drug based on information coming directly from an individual's genome.
Certain alleles associated with a gene will respond more effectively to a specific class of drugs, making knowledge of the allelic combination of a patient beneficial to the physician.
Testing the Fetus
Ultrasounds can detect serious fetal abnormalities in pregnant women.
DNA of fetal cells can be obtained and tested for genetic defects.
Ultrasound
Ultrasound images are used to evaluate fetal anatomy.
A probe is used to scan the mother's abdomen.
High-frequency sound waves are transmitted by a transducer.
The sound waves are transformed into a picture on a video screen.
The picture shows the fetus inside the uterus.
Ultrasound can determine a fetus's age, size, and the presence of more than one fetus.
Chromosomal abnormalities such as Down syndrome, Edwards syndrome, and Patau syndrome can cause anatomical abnormalities that can be detected by ultrasound by the twentieth week of pregnancy.
Routine ultrasound at this time is considered an essential part of prenatal care.
Many other conditions, such as spina bifida, can be diagnosed by ultrasound.
Spina bifida results when the backbone fails to close properly around the spinal cord during the first month of pregnancy.
Surgery to close a newborn's spine in such a case is generally performed within 24 hours after birth.
Testing Fetal Cells
Fetal cells can be tested for genetic disorders using amniocentesis, chorionic villus sampling, or cell-free fetal DNA testing.
Amniocentesis and chorionic villus sampling are invasive procedures that involve collecting cells from the amniotic sac or placenta, respectively.
Cell-free fetal DNA testing is a non-invasive procedure that involves collecting maternal blood and detecting fetal DNA in the blood.
Fetal cells can also be collected from the mother's blood using a cell sorter.
Only about one of every 70,000 blood cells in a mother's blood are fetal cells, so the polymerase chain reaction (PCR) is used to amplify the DNA from the few cells collected.
The procedure poses no risk to the fetus.
Testing the Embryo and Egg
In vitro fertilization (IVF) is done in laboratory glassware.
Eggs from the prospective mother and sperm from the prospective father are obtained by a physician.
The eggs and sperm are placed in the same receptacle for fertilization.
After IVF, the embryo can be tested.
Prior to IVF, the egg can be tested for genetic defects.
Only normal embryos are transferred to the uterus for further development.
Testing the Embryo
Prospective parents who are carriers of genetic disorders may want assurance that their offspring will be free of the disorder.
Genetic diagnosis of the embryo can provide this assurance.
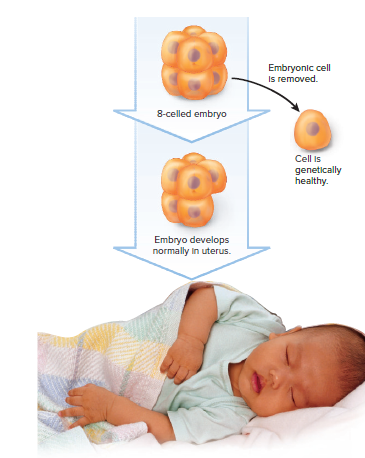
After IVF, when the embryo has six to eight cells, one cell can be removed for diagnosis without affecting normal development.
Only embryos that test negative for the genetic disorders of interest are placed in the uterus to continue developing.
Thousands of children worldwide have been born free of alleles for genetic disorders that run in their families following embryo testing.
In the future, embryos that test positive for a disorder could be treated by gene therapy, allowing them to continue to term.
Testing the Egg
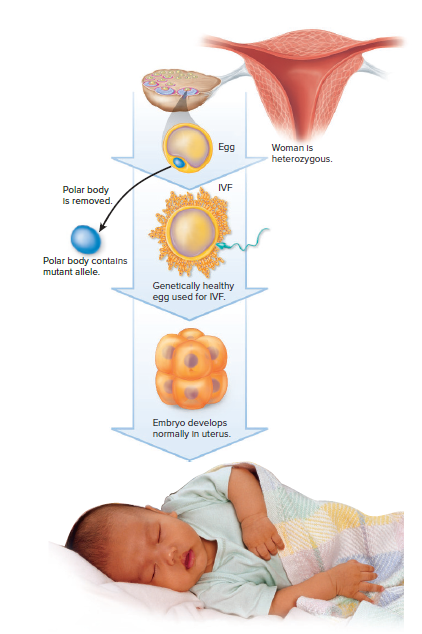
Meiosis in females results in the formation of a single egg and at least two nonfunctional cells called polar bodies.
Polar bodies receive very little cytoplasm, but they do receive a haploid number of chromosomes and thus can be useful for genetic diagnosis.
When a woman is heterozygous for a recessive genetic disorder, about half the polar bodies receive the mutated allele, and in these instances, the egg receives the normal allele.
If a polar body test positive for a mutated allele, the egg probably received the normal allele.
Only normal eggs are then used for IVF.
If gene therapy becomes routine in the future, it’s possible that an egg will be given genes that control traits desired by the parents, such as musical or athletic ability, prior to IVF.
Such genetic manipulation, called eugenics, carries many ethical concerns.
13.4 Gene Therapy
Gene therapy is the insertion of genetic material into human cells for the treatment of a disorder.
It can be used to give a patient healthy genes to make up for faulty genes or to treat various other human illnesses.
Gene therapy can be ex vivo (outside the body) or in vivo (inside the body).
Viruses genetically modified to be safe or liposomes can be used to ferry a normal gene into cells.
Sometimes the gene is injected directly into a particular region of the body.
Ex vivo gene therapy involves inserting the gene into cells that have been removed and then returned to the body.
In vivo, gene therapy involves delivering the gene directly into the body.
Ex Vivo Gene Therapy
Children with SCID lack the ADA enzyme involved in antibody-producing cell maturation.
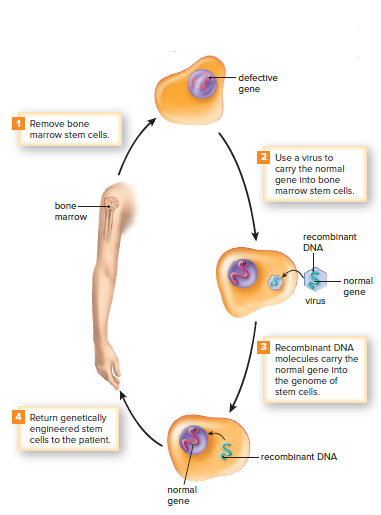
Gene therapy for SCID involves removing bone marrow stem cells, infecting them with a virus carrying a normal ADA gene, and returning them to the patient.
Bone marrow stem cells are preferred because they produce more cells with the same genes.
Patients who undergo this procedure show improved immune function and increased ADA enzyme activity in the blood.
Ex vivo gene therapy is also used for familial hypercholesterolemia by surgically excising a small portion of the liver, infecting it with a retrovirus carrying a normal cholesterol receptor gene, and returning it to the patient.
Patients have experienced lowered plasma cholesterol levels following this procedure.
Ex vivo gene therapy is used for some cancers by removing immune system cells, genetically engineering them to display tumor antigens, and returning them to the patient to stimulate the immune system to kill tumor cells.
In Vivo Gene Therapy
Most cystic fibrosis patients lack a gene that regulates a chloride ion carrier.
Gene therapy trials for cystic fibrosis involve spraying or delivering the needed gene to the respiratory tract.
A combination of adenoviruses, liposomes, and nasal spray may improve gene uptake.
Gene therapy is also used to treat poor coronary circulation by injecting the gene for vascular endothelial growth factor (VEGF) into the heart.
VEGF stimulates the growth of new blood vessels, reducing chest pain and improving exercise tolerance.
In vivo gene therapy has been used to treat rheumatoid arthritis by injecting adenoviruses with an anti-inflammatory gene into the affected joint.
The added gene reduces inflammation and lessens pain and suffering.
Clinical trials have been promising, and animal studies suggest gene therapy may prevent arthritis in at-risk individuals.
Chapter 13: Mutations and Genetic Testing
13.1 Gene Mutations
A gene is a sequence of DNA bases that codes for a cellular product, usually a protein.
Variations in genes are called alleles.
Alleles can code for observable traits like flower color or wing shape or for non-observable traits like susceptibility to medication.
New alleles are formed through mutations or changes in the DNA sequence.
Mutations can have negative consequences, like the alleles for cystic fibrosis.
Mutations can also create new gene products with positive functions for the organism.
Mutations play an important role in the process of evolution.
Causes of Gene Mutations
Gene mutations can be caused by errors in DNA replication, transposons, or environmental mutagens.
Mutations due to DNA replication errors are rare, occurring with a frequency of 1 in 100 million cell divisions on average in most eukaryotes.
DNA polymerase is the enzyme that carries out replication and proofreads the new strand against the old strand, detecting and correcting any mismatched pairs, which contributes to the low frequency of replication errors.
Mutagens are environmental influences that cause mutations, such as different forms of radiation and chemical mutagens, like pesticides and compounds in cigarette smoke.
If mutagens cause a mutation in an individual's gametes, their offspring may be affected. If the mutation occurs in the individual's body cells, cancer may result.
DNA repair enzymes constantly monitor for any irregularity and remedy the problem, contributing to the low overall rate of mutation.
Transposons are specific DNA sequences that can move within and between chromosomes, often disrupting genes and rendering them nonfunctional.
Transposons have been discovered in almost every group of organisms, including bacteria, plants, fruit flies, and humans.

Types and Effects of Mutations
Effects of mutations:
Mutations can have a wide range of effects, from no effect to severe disease.
The severity of a mutation depends on the type of mutation and the gene involved.
Silent mutations are mutations that have no effect on the protein produced by the gene.
Point mutations are mutations that change a single base pair in the DNA.
Frameshift mutations are mutations that add or delete a base pair in the DNA.
Point mutations:
Point mutations can change the amino acid that is coded for by a gene.
Some point mutations are harmless, while others can cause disease.
Sickle cell anemia is a disease that is caused by a point mutation in the gene for hemoglobin.
Frameshift mutations:
Frameshift mutations can change the reading frame of the DNA.
This can cause the protein that is produced by the gene to be nonfunctional.
Fragile X syndrome is a disease that is caused by a frameshift mutation in the FMR1 gene.

13.2 Chromosomal Mutations
Mutations can cause changes in the number of chromosomes or the structure of individual chromosomes.
In humans, variations in chromosome number are typically caused by nondisjunction events during meiosis.
Changes in chromosome structure are more common and can be caused by environmental agents.
Syndromes resulting from changes in chromosome structure are due to the breakage of chromosomes and failure to reunite properly.
Chromosomal mutations can result in deletion, duplication, translocation, or inversion.
Chromosomal mutations can occur during meiosis and may result in a syndrome if the offspring inherits the abnormal chromosome.
Deletions and Duplications
Deletion:
A single break causes the loss of an end piece.
Two simultaneous breaks lead to the loss of an internal segment.
Inheritance of a normal chromosome from one parent and a chromosome with deletion from another parent results in the loss of pair of alleles and can cause the syndrome.
Williams Syndrome:
Occurs when chromosome 7 loses a tiny endpiece.
Symptoms: turned-up nose, wide mouth, small chin, large ears.
Poor academic skills, excellent verbal and musical abilities.
The missing gene for elastin protein affects cardiovascular health and causes premature skin aging.
Friendly but needs ordered life due to loss of gene for a brain protein
Cri du chat Syndrome:
Occurs when chromosome 5 is missing the end piece.
Symptoms: small head, mental disabilities, facial abnormalities.
Abnormal development of the glottis and larynx results in cat-like cries.

Duplication: chromosome segment is repeated, leading to more than two alleles for certain traits.
Inverted duplication: segment runs in the opposite direction from normal.
Occurs in chromosome 15.
Inv dup 15 syndrome: children have poor muscle tone, mental disabilities, seizures, curved spine, and autistic characteristics.
Autistic characteristics include poor speech, hand flapping, and lack of eye contact.

Translocation
A translocation is the movement of a segment from one chromosome to another, a nonhomologous chromosome, or the exchange of segments between nonhomologous chromosomes.
A person who has both of the involved chromosomes has the normal amount of genetic material and a normal phenotype unless the translocation breaks an allele into two pieces or fuses two genes together.
The person who inherits only one of the translocated chromosomes will have only one copy of certain alleles and three copies of other alleles.
A genetic counselor begins to suspect a translocation has occurred when spontaneous abortions are commonplace, and family members suffer from various syndromes.
In 5% of Down syndrome cases, a translocation that occurred in a previous generation between chromosomes 21 and 14 is the cause.
Some forms of cancer are also associated with translocations, such as chronic myeloid leukemia (CML) and Burkitt’s lymphoma.
Alagille syndrome is caused by a translocation between chromosomes 2 and 20, which often produces a deletion on chromosome 20.

Inversion
An inversion occurs when a segment of a chromosome is turned 180° (Fig. 13.6).
The reversed sequence of alleles can lead to altered gene activity if it disrupts the control of gene expression.
Inversions usually do not cause problems, but they can lead to an increased occurrence of abnormal chromosomes during sexual reproduction.
Crossing over between an inverted chromosome and the noninverted homologue can lead to recombinant chromosomes that have both duplicated and deleted segments.
Alignment between the two homologs is only possible when the inverted chromosome forms a loop (Fig. 13.6).

13.3 Genetic Testing
Potential parents are becoming aware of the link between abnormal chromosomal inheritance or gene mutations and illnesses.
More couples are seeking genetic counseling to determine the risk of inherited disorders in their family.
Couples may seek counseling after experiencing several miscarriages, having relatives with a medical condition, or having a child with a genetic defect.
The counselor helps the couple understand the mode of inheritance, the medical consequences of a genetic disorder, and their options.
Abnormal chromosome numbers or structures can result in various human disorders.
If a pregnant woman is concerned about a chromosomal defect in her unborn child, the counselor may recommend karyotyping the fetus's chromosomes.
Analyzing the Chromosomes
A karyotype is a visual display of pairs of chromosomes arranged by size, shape, and banding pattern.
Any cell in the body except red blood cells can be a source of chromosomes for karyotyping.
White blood cells separated from a blood sample are the easiest to use for karyotyping in adults.
Cells can be obtained by either amniocentesis or chorionic villus sampling in fetuses.
The karyotype of a person with Down syndrome usually has three copies of chromosome number 21 instead of two.
Amniocentesis is a procedure for obtaining a sample of amniotic fluid from the uterus of a pregnant woman.
Chorionic villus sampling (CVS) is a procedure for obtaining chorionic villi cells in the region where the placenta will develop.
After a cell sample has been obtained, the cells are stimulated to divide in a culture medium.
Stains are applied to the slides, and the cells are photographed to create a traditional karyotype.
Following genetic testing, a genetic counselor can explain to prospective parents the chances that a child of theirs will have a disorder that runs in the family.


Testing for a Protein
Genetic mutations can cause disorders due to a lack of enzyme activity.
Methemoglobinemia is an example of such a disorder.
Diaphorase enzyme quantity in blood samples can determine if an individual is a homozygous normal carrier or has methemoglobinemia.
If parents are carriers, each child has a 25% chance of having methemoglobinemia.
Prospective parents may opt for embryo or egg testing based on this knowledge.
Testing DNA
Methods of analyzing DNA for specific mutations:
Testing for a genetic marker.
Using a DNA microarray.
Direct sequencing of an individual's DNA.
Genetic Markers
Testing for a genetic marker relies on a difference in DNA due to the presence of an abnormal allele.
Individuals with sickle-cell disease or Huntington's disease have an abnormality in a gene's base sequence, which serves as a genetic marker.
DNA sequencing can detect the presence of specific genetic markers.
Restriction enzymes can be used to cleave DNA at particular base sequences.
The fragments resulting from the use of a restriction enzyme may differ for people who are normal than for those who are heterozygous or homozygous for a mutation.

DNA Microarrays
New technologies have made DNA testing easy and inexpensive.
DNA microarray or gene chip is a small silicon chip containing many DNA samples.
Thousands of known disease-associated mutant alleles can be placed onto a DNA microarray.
Genomic DNA from the subject to be tested is labeled with a fluorescent dye and added to the microarray.
The spots on the microarray fluoresce if the DNA binds to the mutant alleles on the chip, indicating that the subject may have a particular disorder or is at risk of developing it later in life.
An individual’s complete genotype, including all the various mutations, is called a genetic profile.
With the help of a genetic counselor, individuals can be educated about their genetic profile.
A genetic profile can indicate an increased or decreased risk for a disorder.
Risk information can be used to design a program of medical surveillance and to foster a lifestyle aimed at reducing the risk.
For example, an individual with mutations common to people with colon cancer should have an annual colonoscopy to detect and remove any abnormal growths before they become invasive.

DNA Sequencing
Recent advances in DNA sequencing have made it more economically feasible to sequence an individual's genome to detect specific mutations associated with a disease.
The cost of sequencing an individual's genome has decreased from $100,000 to almost $1,000, ushering in an era of personal genomics or personalized medicine.
Personal genomics can be approached by sequencing the entire genome of an individual or targeting specific genes and looking for alleles that increase the risk associated with a specific disease.
Genome-wide association study has become more prevalent in personalized medicine due to the increase in large genomic population studies.
Pharmacogenomics is the selection of a drug based on information coming directly from an individual's genome.
Certain alleles associated with a gene will respond more effectively to a specific class of drugs, making knowledge of the allelic combination of a patient beneficial to the physician.
Testing the Fetus
Ultrasounds can detect serious fetal abnormalities in pregnant women.
DNA of fetal cells can be obtained and tested for genetic defects.
Ultrasound
Ultrasound images are used to evaluate fetal anatomy.
A probe is used to scan the mother's abdomen.
High-frequency sound waves are transmitted by a transducer.
The sound waves are transformed into a picture on a video screen.
The picture shows the fetus inside the uterus.
Ultrasound can determine a fetus's age, size, and the presence of more than one fetus.
Chromosomal abnormalities such as Down syndrome, Edwards syndrome, and Patau syndrome can cause anatomical abnormalities that can be detected by ultrasound by the twentieth week of pregnancy.
Routine ultrasound at this time is considered an essential part of prenatal care.
Many other conditions, such as spina bifida, can be diagnosed by ultrasound.
Spina bifida results when the backbone fails to close properly around the spinal cord during the first month of pregnancy.
Surgery to close a newborn's spine in such a case is generally performed within 24 hours after birth.
Testing Fetal Cells
Fetal cells can be tested for genetic disorders using amniocentesis, chorionic villus sampling, or cell-free fetal DNA testing.
Amniocentesis and chorionic villus sampling are invasive procedures that involve collecting cells from the amniotic sac or placenta, respectively.
Cell-free fetal DNA testing is a non-invasive procedure that involves collecting maternal blood and detecting fetal DNA in the blood.
Fetal cells can also be collected from the mother's blood using a cell sorter.
Only about one of every 70,000 blood cells in a mother's blood are fetal cells, so the polymerase chain reaction (PCR) is used to amplify the DNA from the few cells collected.
The procedure poses no risk to the fetus.
Testing the Embryo and Egg
In vitro fertilization (IVF) is done in laboratory glassware.
Eggs from the prospective mother and sperm from the prospective father are obtained by a physician.
The eggs and sperm are placed in the same receptacle for fertilization.
After IVF, the embryo can be tested.
Prior to IVF, the egg can be tested for genetic defects.
Only normal embryos are transferred to the uterus for further development.
Testing the Embryo
Prospective parents who are carriers of genetic disorders may want assurance that their offspring will be free of the disorder.
Genetic diagnosis of the embryo can provide this assurance.
After IVF, when the embryo has six to eight cells, one cell can be removed for diagnosis without affecting normal development.
Only embryos that test negative for the genetic disorders of interest are placed in the uterus to continue developing.
Thousands of children worldwide have been born free of alleles for genetic disorders that run in their families following embryo testing.
In the future, embryos that test positive for a disorder could be treated by gene therapy, allowing them to continue to term.

Testing the Egg
Meiosis in females results in the formation of a single egg and at least two nonfunctional cells called polar bodies.
Polar bodies receive very little cytoplasm, but they do receive a haploid number of chromosomes and thus can be useful for genetic diagnosis.
When a woman is heterozygous for a recessive genetic disorder, about half the polar bodies receive the mutated allele, and in these instances, the egg receives the normal allele.
If a polar body test positive for a mutated allele, the egg probably received the normal allele.
Only normal eggs are then used for IVF.
If gene therapy becomes routine in the future, it’s possible that an egg will be given genes that control traits desired by the parents, such as musical or athletic ability, prior to IVF.
Such genetic manipulation, called eugenics, carries many ethical concerns.

13.4 Gene Therapy
Gene therapy is the insertion of genetic material into human cells for the treatment of a disorder.
It can be used to give a patient healthy genes to make up for faulty genes or to treat various other human illnesses.
Gene therapy can be ex vivo (outside the body) or in vivo (inside the body).
Viruses genetically modified to be safe or liposomes can be used to ferry a normal gene into cells.
Sometimes the gene is injected directly into a particular region of the body.
Ex vivo gene therapy involves inserting the gene into cells that have been removed and then returned to the body.
In vivo, gene therapy involves delivering the gene directly into the body.
Ex Vivo Gene Therapy
Children with SCID lack the ADA enzyme involved in antibody-producing cell maturation.
Gene therapy for SCID involves removing bone marrow stem cells, infecting them with a virus carrying a normal ADA gene, and returning them to the patient.
Bone marrow stem cells are preferred because they produce more cells with the same genes.
Patients who undergo this procedure show improved immune function and increased ADA enzyme activity in the blood.
Ex vivo gene therapy is also used for familial hypercholesterolemia by surgically excising a small portion of the liver, infecting it with a retrovirus carrying a normal cholesterol receptor gene, and returning it to the patient.
Patients have experienced lowered plasma cholesterol levels following this procedure.
Ex vivo gene therapy is used for some cancers by removing immune system cells, genetically engineering them to display tumor antigens, and returning them to the patient to stimulate the immune system to kill tumor cells.

In Vivo Gene Therapy
Most cystic fibrosis patients lack a gene that regulates a chloride ion carrier.
Gene therapy trials for cystic fibrosis involve spraying or delivering the needed gene to the respiratory tract.
A combination of adenoviruses, liposomes, and nasal spray may improve gene uptake.
Gene therapy is also used to treat poor coronary circulation by injecting the gene for vascular endothelial growth factor (VEGF) into the heart.
VEGF stimulates the growth of new blood vessels, reducing chest pain and improving exercise tolerance.
In vivo gene therapy has been used to treat rheumatoid arthritis by injecting adenoviruses with an anti-inflammatory gene into the affected joint.
The added gene reduces inflammation and lessens pain and suffering.
Clinical trials have been promising, and animal studies suggest gene therapy may prevent arthritis in at-risk individuals.
 Knowt
Knowt
