
Chapter 5: Formations of Ions and Bonding
5.1-Formations of Ions
Ions are made when electrons are transferred
Ions are charged particles-they can be single atoms or groups of atoms
When atoms lose of gain electrons to form ions, all they’re trying to do is get a full outer shell like a noble gas
Atoms with full outer shell are very stable
When metals form ions, they lose electrons from their outer shell to form positive ions
When non-metals form ions, they gain electrons into their outer shell to form negative ions
The number of electrons lost or gained is the same as the charge on the ion
E.g. If 2 electrons are lost the charge is 2+.
If 3 electrons are gained the charge is 3-
Groups 1&2 and 6&7 are the most likely to form ions
The elements that most readily form ions are those in Group 1,2,6 and 7
Group 1 and 2 elements are metals and they lose electrons to form positive ions(cations)
Group 6 and 7 elements are non-metals and they gain electrons to form negative ions(anions)
You don’t have to remember what ions most elements form
You just look at the periodic table
Elements in the same group all have the same number of outer electrons
So they have to lose or gain the same number to get a full outer shell
This means that they form ions with the same charges
Group 1 elements form 1+ ions
Group 2 elements form 2+ ions
Group 6 elements form 2- ions
Group 7 elements form 1- ions
Examples of formation:
A sodium atom is in Group 1 so it loses 1 electron to form a sodium ion with the same electronic structure as a neon
Na-Na+ + e-
A magnesium atom is in Group 2 so it loses 2 electrons to form a magnesium ion with the same electronic structure as neon
Mg-Mg2+ + 2e-
A chlorine atom is in Group 7 so it gains 1 electron to form a chloride ion with the same electronic structure as argon
Cl + e- - cl-
An oxygen atom is in Group 6 so it gains 2 electrons to form an oxide ion with the same electronic structure as neon
O + 2e- - O2-
5.2-Ionic Bonding
Ionic bonding-transfer of electrons
When a metal and a non-metal react together, the metal atom loses electrons to form a positively charged ion and the non-metal gains these electrons to form a negatively charged ion
These oppositely charged ions are strongly attracted to one another by electrostatic forces
This attraction is called an ionic bond
Dot and cross diagrams show how ionic compounds are formed
Dot and cross diagrams show the arrangement of electrons in an atom or ion
Each electron is represented by a dot or a cross
So these diagrams can show which atom the electrons in an ion originally came from
Sodium chloride
The sodium atom gives up its outer electron, becoming an Na+ ion
The chlorine atom picks up the electron, becoming a Cl- ion
Magnesium chloride
The magnesium atom gives up its two outer electrons, becoming an Mg2+ ion
The two chlorine atoms picks up one electron each, becoming two Cl- ions
Sodium oxide
Two sodium atoms each give up their single outer electron, becoming two Na+ ions
The oxygen atom picks up the two electrons, becoming an 02- ion
Dot and cross diagrams are useful for showing how ionic compounds are formed, but they don’t show the structure of the compounds, the size of the ions or how they’re arranged.
5.3-Ionic Compounds
Ionic compounds have a regular lattice structure
Ionic compounds have a structure called a giant ionic lattice
The ions form a closely packed regular lattice arrangement and there are very strong electrostatic forces of attraction between oppositely charged ions
In all directions in the lattice
A single crystal of sodium chloride(table salt) is one giant ionic lattice
The Na+ and Cl- ions are held together in a regular lattice
The lattice can be represented in different ways:
A model could show the relative sizes of the ions as well as the regular pattern of an ionic crystal, but it only lets you see the outer layer of the compound
A model could show a ball and stick model
It would show the regular pattern of an ionic crystal and show how all the ions are arranged
It also suggest that the crystal extend beyond what’s shown in the diagram
The model isn’t to scale so the relative sizes of the ions may not be shown
Also in reality there aren’t gaps between the ions
Ionic compounds all have similar properties
They all have high melting and boiling points due to the many strong bonds between the ions
It tales lots of energy to overcome this attraction
When they’re solid, the ions are held in place, so the compounds can’t conduct electricity
When ionic compounds melt, the ions are free to move and they’ll carry electric current
Some ionic compounds also dissolve in water
The ions separate and are all free to move in the solution, so they’ll carry electric current
Look at charges to find the formula of an ionic compound
You might have to work out the empirical formula of an ionic compound from a diagram of the compound
If it’s a dot and cross diagram, count up how many atoms there are of each elements
Write this down to give you the empirical formula
If you’re given a 3D diagram of the ionic lattice, use it to work out what ions are in the ionic compound
You’ll then have to balance the charges of the ions so that the overall charge on the compound in zero
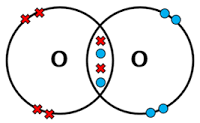
5.4-Covalent Bonding
Covalent Bonds-sharing electrons
When non-metal atoms bond together, they share pairs of electrons to make covalent bonds
The positively charged nuclei of the bonded atoms are attracted to the shared pair of electrons by electrostatic forces, making covalent bonds very strong
Atoms only share electrons in their outer shells
Highest energy levels
Each single covalent bond provides one extra shared electron for each atom
Each atom involved generally makes enough covalent bonds to fill up its outer shell
Having a full outer shell gives them the electronic structure of a noble gas, which is very stable
Covalent bonding happens in compounds of non-metals and in non-metal elements
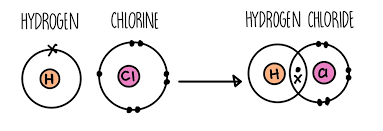
There are different ways of drawing covalent bonds
You can use dot and cross diagrams
You can use 3D models
By using the molecular formula
Chapter 5: Formations of Ions and Bonding
5.1-Formations of Ions
Ions are made when electrons are transferred
Ions are charged particles-they can be single atoms or groups of atoms
When atoms lose of gain electrons to form ions, all they’re trying to do is get a full outer shell like a noble gas
Atoms with full outer shell are very stable
When metals form ions, they lose electrons from their outer shell to form positive ions
When non-metals form ions, they gain electrons into their outer shell to form negative ions
The number of electrons lost or gained is the same as the charge on the ion
E.g. If 2 electrons are lost the charge is 2+.
If 3 electrons are gained the charge is 3-
Groups 1&2 and 6&7 are the most likely to form ions
The elements that most readily form ions are those in Group 1,2,6 and 7
Group 1 and 2 elements are metals and they lose electrons to form positive ions(cations)
Group 6 and 7 elements are non-metals and they gain electrons to form negative ions(anions)
You don’t have to remember what ions most elements form
You just look at the periodic table
Elements in the same group all have the same number of outer electrons
So they have to lose or gain the same number to get a full outer shell
This means that they form ions with the same charges
Group 1 elements form 1+ ions
Group 2 elements form 2+ ions
Group 6 elements form 2- ions
Group 7 elements form 1- ions
Examples of formation:
A sodium atom is in Group 1 so it loses 1 electron to form a sodium ion with the same electronic structure as a neon
Na-Na+ + e-
A magnesium atom is in Group 2 so it loses 2 electrons to form a magnesium ion with the same electronic structure as neon
Mg-Mg2+ + 2e-
A chlorine atom is in Group 7 so it gains 1 electron to form a chloride ion with the same electronic structure as argon
Cl + e- - cl-
An oxygen atom is in Group 6 so it gains 2 electrons to form an oxide ion with the same electronic structure as neon
O + 2e- - O2-
5.2-Ionic Bonding
Ionic bonding-transfer of electrons
When a metal and a non-metal react together, the metal atom loses electrons to form a positively charged ion and the non-metal gains these electrons to form a negatively charged ion
These oppositely charged ions are strongly attracted to one another by electrostatic forces
This attraction is called an ionic bond
Dot and cross diagrams show how ionic compounds are formed
Dot and cross diagrams show the arrangement of electrons in an atom or ion
Each electron is represented by a dot or a cross
So these diagrams can show which atom the electrons in an ion originally came from
Sodium chloride
The sodium atom gives up its outer electron, becoming an Na+ ion
The chlorine atom picks up the electron, becoming a Cl- ion
Magnesium chloride
The magnesium atom gives up its two outer electrons, becoming an Mg2+ ion
The two chlorine atoms picks up one electron each, becoming two Cl- ions
Sodium oxide
Two sodium atoms each give up their single outer electron, becoming two Na+ ions
The oxygen atom picks up the two electrons, becoming an 02- ion
Dot and cross diagrams are useful for showing how ionic compounds are formed, but they don’t show the structure of the compounds, the size of the ions or how they’re arranged.

5.3-Ionic Compounds
Ionic compounds have a regular lattice structure
Ionic compounds have a structure called a giant ionic lattice
The ions form a closely packed regular lattice arrangement and there are very strong electrostatic forces of attraction between oppositely charged ions
In all directions in the lattice
A single crystal of sodium chloride(table salt) is one giant ionic lattice
The Na+ and Cl- ions are held together in a regular lattice
The lattice can be represented in different ways:
A model could show the relative sizes of the ions as well as the regular pattern of an ionic crystal, but it only lets you see the outer layer of the compound
A model could show a ball and stick model
It would show the regular pattern of an ionic crystal and show how all the ions are arranged
It also suggest that the crystal extend beyond what’s shown in the diagram
The model isn’t to scale so the relative sizes of the ions may not be shown
Also in reality there aren’t gaps between the ions
Ionic compounds all have similar properties
They all have high melting and boiling points due to the many strong bonds between the ions
It tales lots of energy to overcome this attraction
When they’re solid, the ions are held in place, so the compounds can’t conduct electricity
When ionic compounds melt, the ions are free to move and they’ll carry electric current
Some ionic compounds also dissolve in water
The ions separate and are all free to move in the solution, so they’ll carry electric current
Look at charges to find the formula of an ionic compound
You might have to work out the empirical formula of an ionic compound from a diagram of the compound
If it’s a dot and cross diagram, count up how many atoms there are of each elements
Write this down to give you the empirical formula
If you’re given a 3D diagram of the ionic lattice, use it to work out what ions are in the ionic compound
You’ll then have to balance the charges of the ions so that the overall charge on the compound in zero
5.4-Covalent Bonding
Covalent Bonds-sharing electrons
When non-metal atoms bond together, they share pairs of electrons to make covalent bonds
The positively charged nuclei of the bonded atoms are attracted to the shared pair of electrons by electrostatic forces, making covalent bonds very strong
Atoms only share electrons in their outer shells
Highest energy levels
Each single covalent bond provides one extra shared electron for each atom
Each atom involved generally makes enough covalent bonds to fill up its outer shell
Having a full outer shell gives them the electronic structure of a noble gas, which is very stable
Covalent bonding happens in compounds of non-metals and in non-metal elements

There are different ways of drawing covalent bonds
You can use dot and cross diagrams
You can use 3D models
By using the molecular formula
 Knowt
Knowt
