
Chapter 7: Chemical Quantities and Reactions
7.1: The Mole
Mole: A group of atoms, molecules, or formula units that contains 6.02 x 10^23 of these items.
Avogadro’s Number:
The number of items in a mole is equal to 6.02 x 10^23.
Named after Amedeo Avogadro, an Italian physicist.
One mole of any element always contains Avogadro’s number of atoms.
Formula Unit: The groups of ions represented by the formula of an ionic compound.
7.2: Molar Mass and Calculations
Molar Mass: The quantity in grams that equals the atomic mass of an element.
The molar mass of an element is useful to convert moles of an element to grams, or grams to moles.
Calculating the Molar Mass of a Compound
Calculate the molar mass for lithium carbonate, Li2CO3, used to produce red color in fireworks.
Step 1: Obtain the molar mass of each element.

Step 2: Multiply each molar mass by the number of moles (subscript) in the formula.
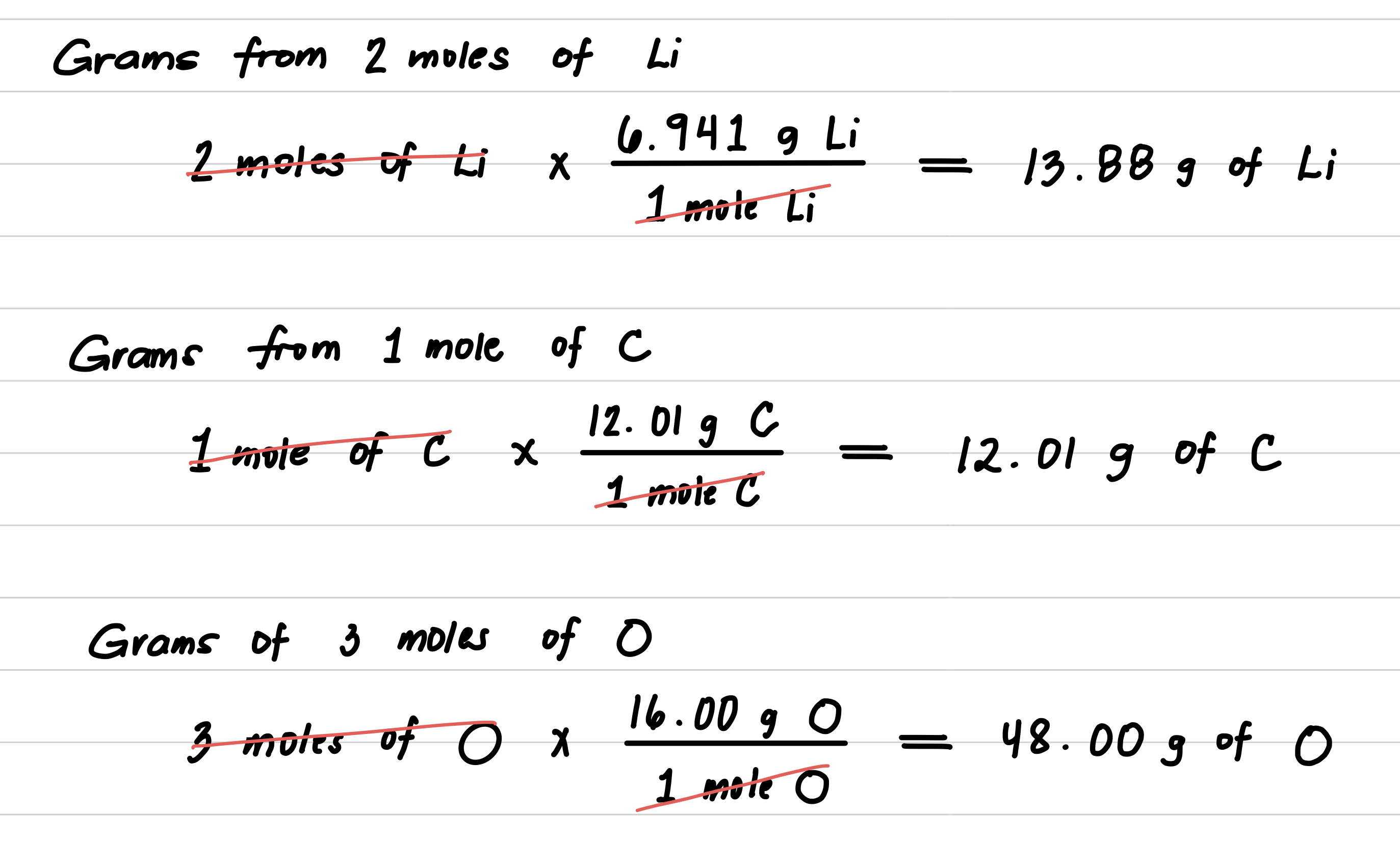
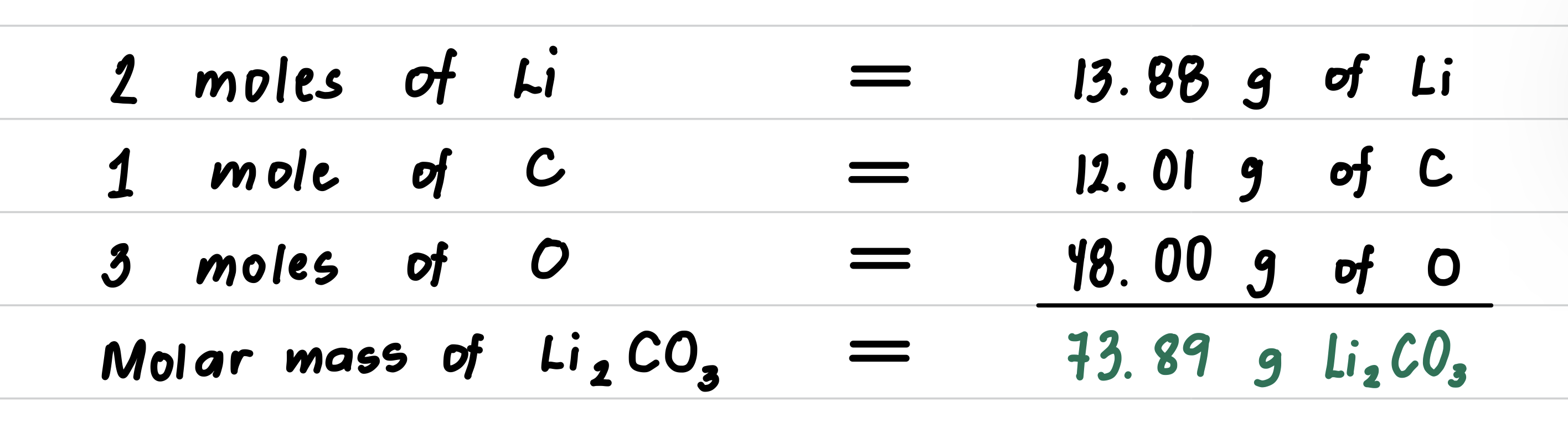
Step 3: Calculate the molar mass by adding the masses of the elements.
Converting Moles of an Element to Grams
Silver metal is used in the manufacture of tableware, mirrors, jewelry, and dental alloys. If the design for a piece of jewelry requires 0.750 mole of silver, how many grams of silver are needed?
Step 1: State the given and needed quantities.
Step 2: Write a plan to convert moles to grams.
Step 3: Determine the molar mass and write conversion factors.
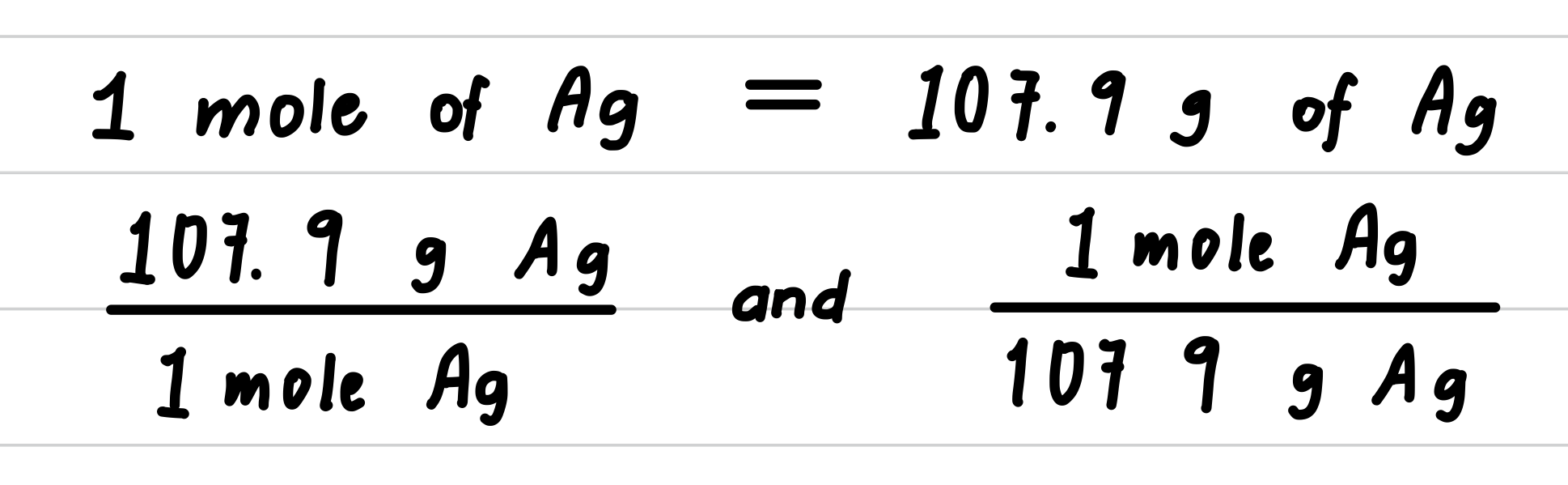
Step 4: Set up the problem to convert moles to grams.
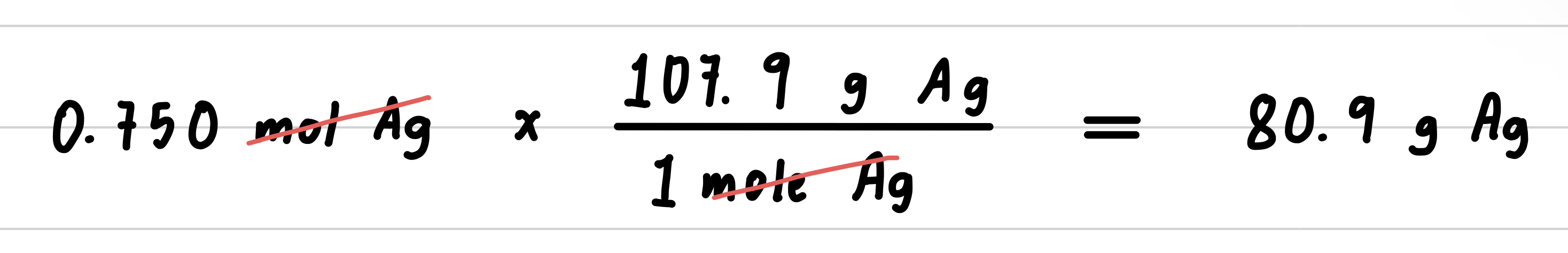
Converting the Mass of a Compound to Moles
A box of salt contains 737 g of Sodium Chloride (NaCl). How many moles of NaCl are present?
Step 1: State the given and needed quantities.
Step 2: Write a plan to convert grams to moles.
Step 3: Determine the molar mass and write conversion factors.
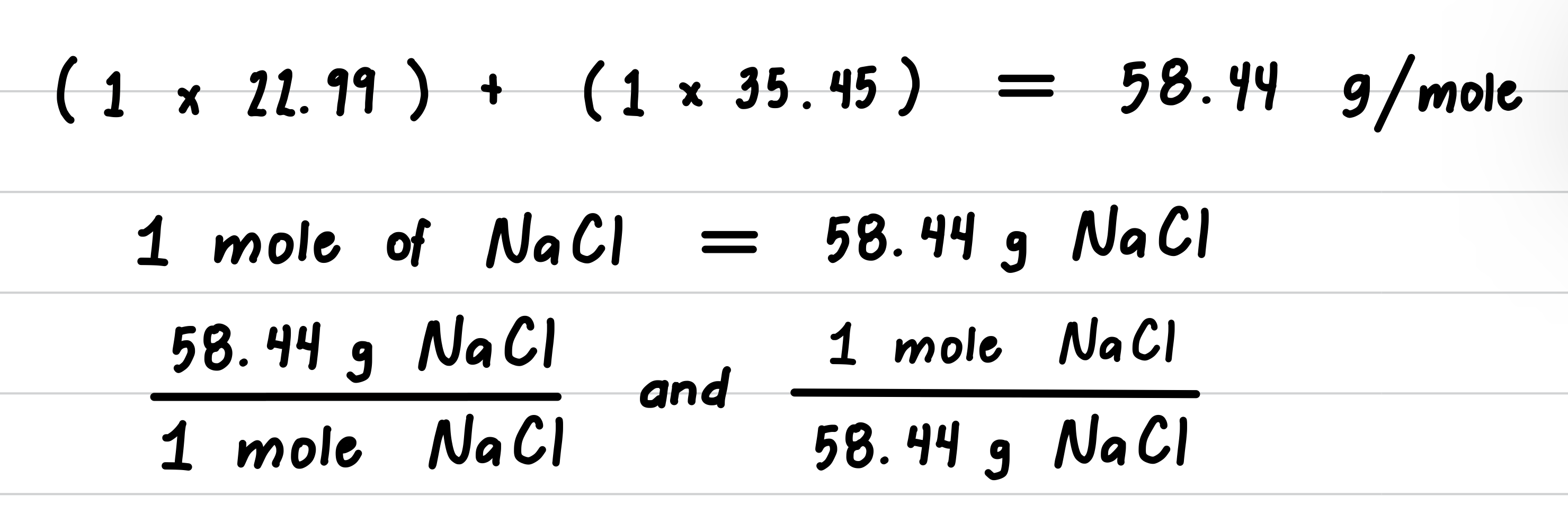
Step 4: Set up the problem to convert grams to moles.

7.3: Equations for Chemical Reactions
Chemical Change: It occurs when a substance is converted into one or more new substances that have different formulas and different properties
Chemical Reactions: These always involve chemical change because atoms of the reacting substances form new combinations with new properties.
Chemical Equation: It tells us the materials we need and the products that will form.
Here, the formulas of the reactants are written on the left of the arrow, and the formulas of the products are on the right
When there are two or more formulas on the same side, they are separated by a plus (+) sign. The delta sign (∆) indicates that heat was used to start the reaction.
Balanced Equations: These show the same number of atoms for each element in the reactants as well as in the products.
Coefficients: The whole numbers in front of the formulas.
Law of Conservation of Matter: States that matter cannot be created or destroyed during a chemical reaction.
Balancing a Chemical Equation
The chemical reaction of methane, CH4, and oxygen gas, O2, produces carbon dioxide (CO2) and water (H2O). Write a balanced chemical equation for this reaction.
Step 1: Write an equation using the correct formulas for the reactants and products.

Step 2: Count the atoms of each element in the reactants and products.

Step 3: Use coefficients to balance each element.
By placing a coefficient of 2 in front of the formula for H2O, a total of 4 H atoms in the products is obtained.
We can balance the O atoms on the reactant side by placing a coefficient of 2 in front of the formula O2. There are now 4 O atoms in both the reactants and products.

Step 4: Check the final equation to confirm it is balanced.
In the final equation, the numbers of atoms of C, H, and O are the same in both the reactants and the products. The equation is balanced.
In a balanced chemical equation, the coefficients must be the lowest possible whole numbers. Suppose you had obtained the following for the balanced equation:

To obtain coefficients that are the lowest whole numbers, we divide all the coefficients by 2.
7.4: Types of Reactions
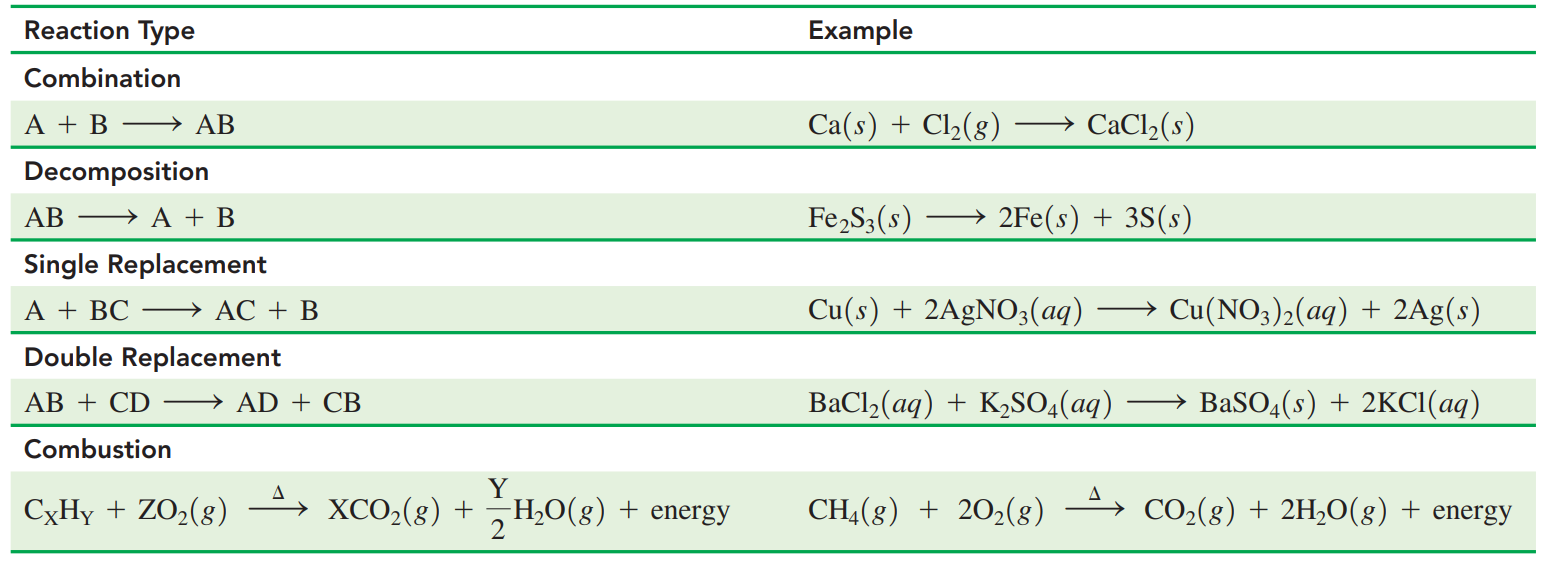
Combination Reactions: Reaction from two or more elements or compounds bond that forms one product.
Decomposition Reactions: These occur when a reactant splits into two or simpler products.
Replacement Reactions: The element in a compound is replaced by other elements.
Single replacement reaction: A reacting element switches place with an element in the other reacting compound.
Double replacement reaction: The positive ions in the reacting compounds switch places.
Combustion Reactions: A carbon-containing compound, usually a fuel burns in oxygen from the air to produce carbon dioxide, water, and energy in the form of heat or flame.
7.5: Oxidation-Reduction Reactions
Oxidation-Reduction Reactions
Electrons are transferred from one substance to another.
If one substance loses electrons, another substance must gain electrons.
Oxidation: The loss of electrons.
Reduction: The gain of electrons.
In general, atoms of metals lose electrons to form positive ions, whereas nonmetals gain electrons to form negative ions.
In the cells of the body, the oxidation of organic (carbon) compounds involves the transfer of hydrogen atoms, which are composed of electrons and protons.
In many biochemical oxidation–reduction reactions, the transfer of hydrogen atoms is necessary for the production of energy in the cells.
7.6: Mole Relationships in Chemical Equations
Law of Conservation of Mass: States that there is no change in the total mass of the substances reacting in a chemical reaction.
In a balanced equation, the total mass of the reactants is equal to the total mass of the products.
The coefficients in an equation describing the relationship between the moles of any two components are used to write mole–mole factors.
When the number of moles for one substance is known, a mole–mole factor is used to find the moles of a different substance in the reaction.
7.7: Mass Calculations for Reactions
When we have the balanced chemical equation for a reaction, we can use the mass of one of the substances (A) in the reaction to calculate the mass of another substance (B) in the reaction.
The calculations require us to convert the mass of A to moles of A using the molar mass factor for A.
Then we use the mole–mole factor that links substance A to substance B, which we obtain from the coefficients in the balanced equation.

7.8: Energy in Chemical Reactions
Activation Energy: The amount of energy required to break the bonds between atoms of the reactants.
Three Conditions Required for a Reaction to Occur
Collision: The reactants must collide
Orientation: The reactants must align properly to break and form bonds.
Energy: The collision must provide the energy of activation.
Heat of reaction: It is the difference between the energy of the reactants and the energy of the products.
Exothermic Reaction: The energy of the products is lower than the energy of the reactants.
Endothermic Reaction: The energy of the products is higher than that of the reactants.
Rate of Reaction: It is measured by the amount of reactant used up, or the amount of product formed, in a certain period.
Reactions with low activation energies go faster than reactions with high activation energies.
Catalyst: It acts by providing an alternate pathway with a lower energy requirement.
Chapter 7: Chemical Quantities and Reactions
7.1: The Mole
Mole: A group of atoms, molecules, or formula units that contains 6.02 x 10^23 of these items.
Avogadro’s Number:
The number of items in a mole is equal to 6.02 x 10^23.
Named after Amedeo Avogadro, an Italian physicist.
One mole of any element always contains Avogadro’s number of atoms.
Formula Unit: The groups of ions represented by the formula of an ionic compound.
7.2: Molar Mass and Calculations
Molar Mass: The quantity in grams that equals the atomic mass of an element.
The molar mass of an element is useful to convert moles of an element to grams, or grams to moles.
Calculating the Molar Mass of a Compound
Calculate the molar mass for lithium carbonate, Li2CO3, used to produce red color in fireworks.
Step 1: Obtain the molar mass of each element.

Step 2: Multiply each molar mass by the number of moles (subscript) in the formula.

Step 3: Calculate the molar mass by adding the masses of the elements.

Converting Moles of an Element to Grams
Silver metal is used in the manufacture of tableware, mirrors, jewelry, and dental alloys. If the design for a piece of jewelry requires 0.750 mole of silver, how many grams of silver are needed?
Step 1: State the given and needed quantities.
Step 2: Write a plan to convert moles to grams.
Step 3: Determine the molar mass and write conversion factors.
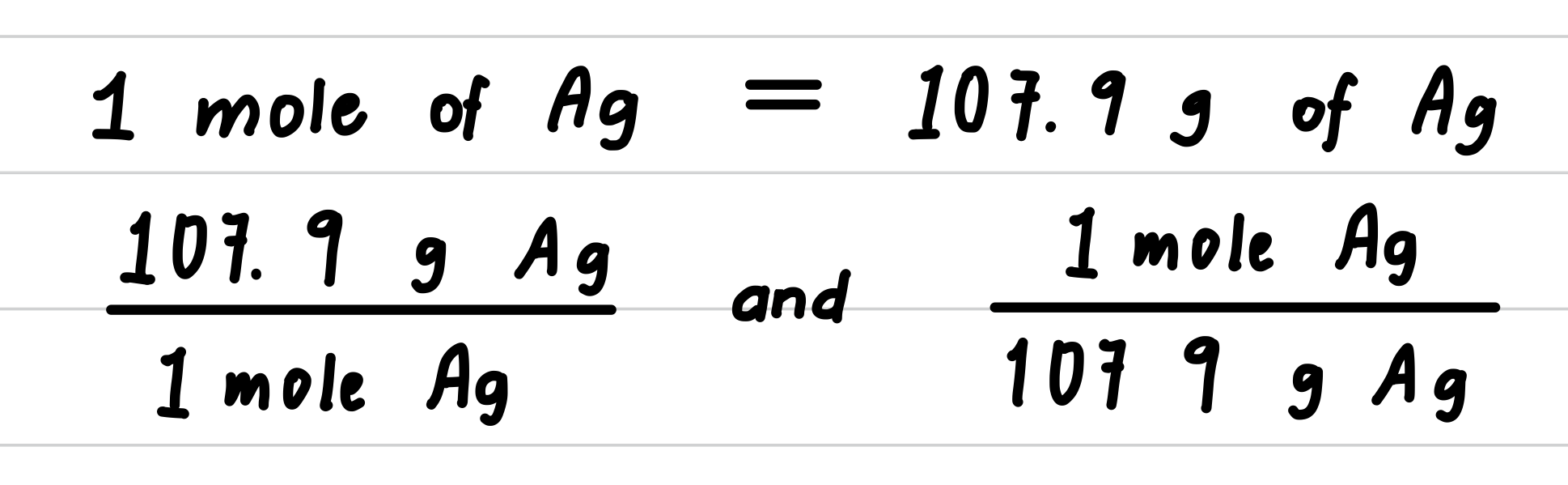
Step 4: Set up the problem to convert moles to grams.
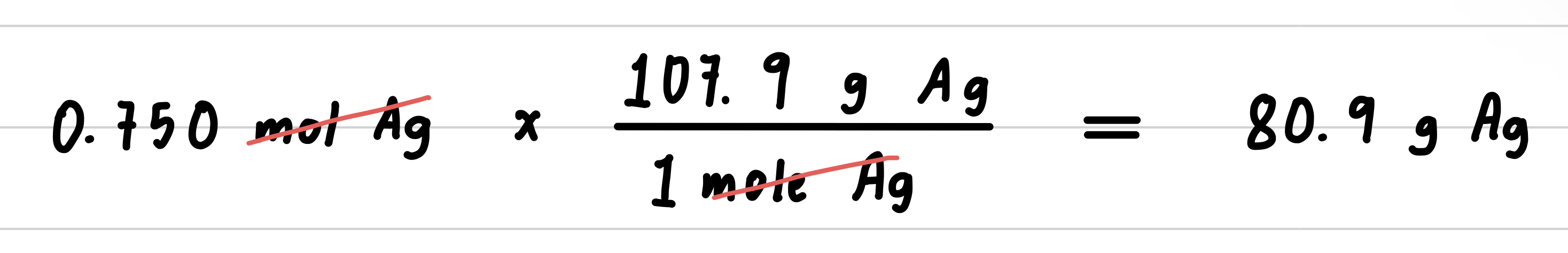
Converting the Mass of a Compound to Moles
A box of salt contains 737 g of Sodium Chloride (NaCl). How many moles of NaCl are present?
Step 1: State the given and needed quantities.
Step 2: Write a plan to convert grams to moles.
Step 3: Determine the molar mass and write conversion factors.
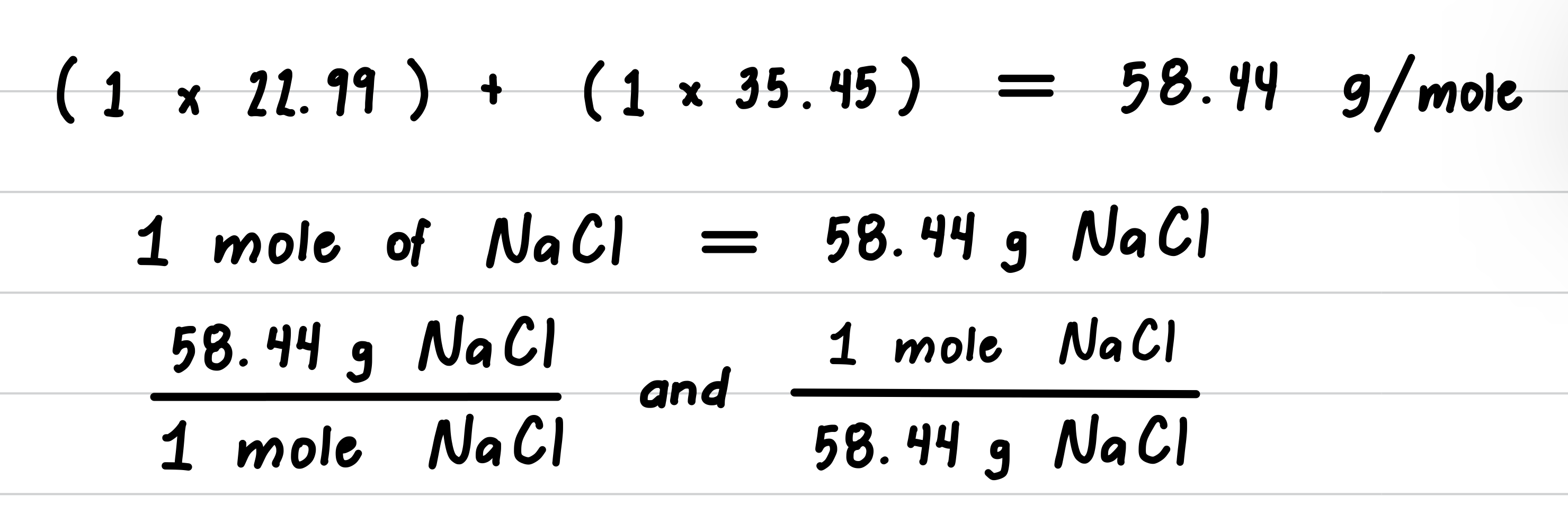
Step 4: Set up the problem to convert grams to moles.

7.3: Equations for Chemical Reactions
Chemical Change: It occurs when a substance is converted into one or more new substances that have different formulas and different properties
Chemical Reactions: These always involve chemical change because atoms of the reacting substances form new combinations with new properties.
Chemical Equation: It tells us the materials we need and the products that will form.
Here, the formulas of the reactants are written on the left of the arrow, and the formulas of the products are on the right
When there are two or more formulas on the same side, they are separated by a plus (+) sign. The delta sign (∆) indicates that heat was used to start the reaction.
Balanced Equations: These show the same number of atoms for each element in the reactants as well as in the products.
Coefficients: The whole numbers in front of the formulas.
Law of Conservation of Matter: States that matter cannot be created or destroyed during a chemical reaction.
Balancing a Chemical Equation
The chemical reaction of methane, CH4, and oxygen gas, O2, produces carbon dioxide (CO2) and water (H2O). Write a balanced chemical equation for this reaction.
Step 1: Write an equation using the correct formulas for the reactants and products.

Step 2: Count the atoms of each element in the reactants and products.

Step 3: Use coefficients to balance each element.
By placing a coefficient of 2 in front of the formula for H2O, a total of 4 H atoms in the products is obtained.
We can balance the O atoms on the reactant side by placing a coefficient of 2 in front of the formula O2. There are now 4 O atoms in both the reactants and products.

Step 4: Check the final equation to confirm it is balanced.
In the final equation, the numbers of atoms of C, H, and O are the same in both the reactants and the products. The equation is balanced.
In a balanced chemical equation, the coefficients must be the lowest possible whole numbers. Suppose you had obtained the following for the balanced equation:

To obtain coefficients that are the lowest whole numbers, we divide all the coefficients by 2.
7.4: Types of Reactions
Combination Reactions: Reaction from two or more elements or compounds bond that forms one product.
Decomposition Reactions: These occur when a reactant splits into two or simpler products.
Replacement Reactions: The element in a compound is replaced by other elements.
Single replacement reaction: A reacting element switches place with an element in the other reacting compound.
Double replacement reaction: The positive ions in the reacting compounds switch places.
Combustion Reactions: A carbon-containing compound, usually a fuel burns in oxygen from the air to produce carbon dioxide, water, and energy in the form of heat or flame.

7.5: Oxidation-Reduction Reactions
Oxidation-Reduction Reactions
Electrons are transferred from one substance to another.
If one substance loses electrons, another substance must gain electrons.
Oxidation: The loss of electrons.
Reduction: The gain of electrons.
In general, atoms of metals lose electrons to form positive ions, whereas nonmetals gain electrons to form negative ions.
In the cells of the body, the oxidation of organic (carbon) compounds involves the transfer of hydrogen atoms, which are composed of electrons and protons.
In many biochemical oxidation–reduction reactions, the transfer of hydrogen atoms is necessary for the production of energy in the cells.
7.6: Mole Relationships in Chemical Equations
Law of Conservation of Mass: States that there is no change in the total mass of the substances reacting in a chemical reaction.
In a balanced equation, the total mass of the reactants is equal to the total mass of the products.
The coefficients in an equation describing the relationship between the moles of any two components are used to write mole–mole factors.
When the number of moles for one substance is known, a mole–mole factor is used to find the moles of a different substance in the reaction.
7.7: Mass Calculations for Reactions
When we have the balanced chemical equation for a reaction, we can use the mass of one of the substances (A) in the reaction to calculate the mass of another substance (B) in the reaction.
The calculations require us to convert the mass of A to moles of A using the molar mass factor for A.
Then we use the mole–mole factor that links substance A to substance B, which we obtain from the coefficients in the balanced equation.

7.8: Energy in Chemical Reactions
Activation Energy: The amount of energy required to break the bonds between atoms of the reactants.
Three Conditions Required for a Reaction to Occur
Collision: The reactants must collide
Orientation: The reactants must align properly to break and form bonds.
Energy: The collision must provide the energy of activation.
Heat of reaction: It is the difference between the energy of the reactants and the energy of the products.
Exothermic Reaction: The energy of the products is lower than the energy of the reactants.
Endothermic Reaction: The energy of the products is higher than that of the reactants.
Rate of Reaction: It is measured by the amount of reactant used up, or the amount of product formed, in a certain period.
Reactions with low activation energies go faster than reactions with high activation energies.
Catalyst: It acts by providing an alternate pathway with a lower energy requirement.
 Knowt
Knowt
