
Chapter 3: Matter and Energy
3.1: Classification of Matter
Matter: Anything that has mass and occupies space.
Pure Substances: A matter that has a fixed or definite composition.
Element: The simplest type of pure substance. It is composed of only one type of material.
Atoms: These are extremely tiny particles that make up each type of matter.
Compound: A pure substance that consists of atoms of two or more elements, always chemically combined in the same proportion.
Bonds: Happens when atoms are held together by attractions.
Molecules: Small groups of atoms.
Pure substances that are compounds can be broken down by chemical processes into their elements. They cannot be broken down through physical methods.
In a mixture, two or more different substances are physically mixed, but not chemically combined.
Physical processes can be used to separate mixtures because there are no chemical interactions between the components.
Homogenous Mixture: Also called a solution, the composition is uniform throughout the sample.
Heterogeneous Mixture: The components do not have a uniform composition throughout the sample.
Filtration: It helps in separating solids from liquids, which involves pouring a mixture through a filter paper set in a funnel.
Chromatography: Different components of a liquid mixture separate as they move at different rates up the surface of a piece of chromatography paper.
3.2: States and Properties of Matter
State of Matter: The physical forms of matter.
Solid: It has a definite shape and volume.
Strong attractive forces hold the particles such as atoms or molecules close together.
The particles here are arranged in such a rigid pattern, their only movement is to vibrate slowly in fixed positions.
Liquid: It has a definite volume, but not a definite shape.
The particles here move in random directions but are sufficiently attracted to each other to maintain a definite volume, although not a rigid structure.
Gas: It does not have a definite shape or volume.
The particles here are far apart, have little attraction to each other, and move at high speeds, taking the shape and volume of their container.
Physical properties
These are those characteristics that can be observed or measured without affecting the identity of a substance.
When matter undergoes a physical change, its state, size, or appearance will change, but its composition remains the same.
Examples:
Water boils to form water vapor.
Sugar dissolves in water to form a solution.
Copper is drawn into thin copper wires.
Paper is cut into tiny pieces of confetti.
Pepper is ground into flakes.
Chemical properties
These are those that describe the ability of a substance to change into a new substance.
When a chemical change takes place, the original substance is converted into one or more new substances, which have different physical and chemical properties.
Examples:
Shiny, silver metal reacts in air to give a black, grainy coating.
A piece of wood burns with a bright flame, and produces heat, ashes, carbon dioxide, and water vapor.
Heating white, granular sugar forms a smooth, caramel-colored substance.
Iron, which is gray and shiny, combines with oxygen to form orange-red rust.
3.3: Temperature
Temperatures in science, are measured and reported in Celsius (°C) units.
The freezing point of water, is defined as 0 °C, and the boiling point is 100 °C.
In the United States, everyday temperatures are commonly reported in Fahrenheit (°F) units.
Water freezes at exactly 32 °F and boils at 212 °F.
A typical room temperature of 22 °C would be the same as 72 °F.
Normal human body temperature is 37.0 °C, which is the same temperature as 98.6 °C.
Degrees: Smaller units of temperature.
Scientists have learned that the coldest temperature possible is 273 °C.
On the Kelvin scale, 273 °C temperature, which is called absolute zero, and has the value of 0 K.
Kelvins (K): The unit of the Kelvin Scale, no degree symbol is used.
3.4: Energy
Energy: The ability to do work.
Kinetic Energy: The energy of motion.
Potential Energy: Determined by the position of an object or by the chemical composition of a substance.
Heat: The energy associated with the motion of particles.
Joule (J): The SI unit of energy and work.
It is a small amount of energy and scientists often use the kilojoule (kJ).
Calorie (cal): Defined as the amount of energy needed to raise the temperature of 1 g of water by 1 °C.
3.5: Energy and Nutrition
Carbohydrates are the primary fuel for the body.
If the carbohydrate reserves are exhausted, fats and then proteins are used for energy.
In the nutrition laboratory, foods are burned in a calorimeter to determine their energy value.
The energy values for food are the kilocalories or kilojoules obtained from burning 1 g of carbohydrate, fat, or protein.
3.6: Specific Heat
Specific Heat
The amount of heat needed to raise the temperature of exactly 1 g of a substance by exactly 1 °C.
This temperature change is written as ∆T (delta T), where the delta symbol means “change in.”
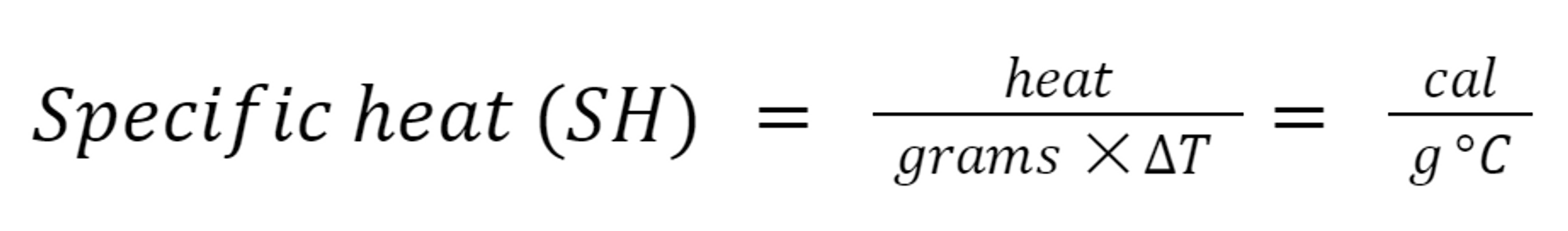
The high specific heat of the water has a major impact on the temperatures in a coastal city compared to an inland city.
A large mass of water near a coastal city can absorb or release five times the energy absorbed or released by the same mass of rock near an inland city.
Heat Equation: Specific heat expression that is arranged to solve for heat.
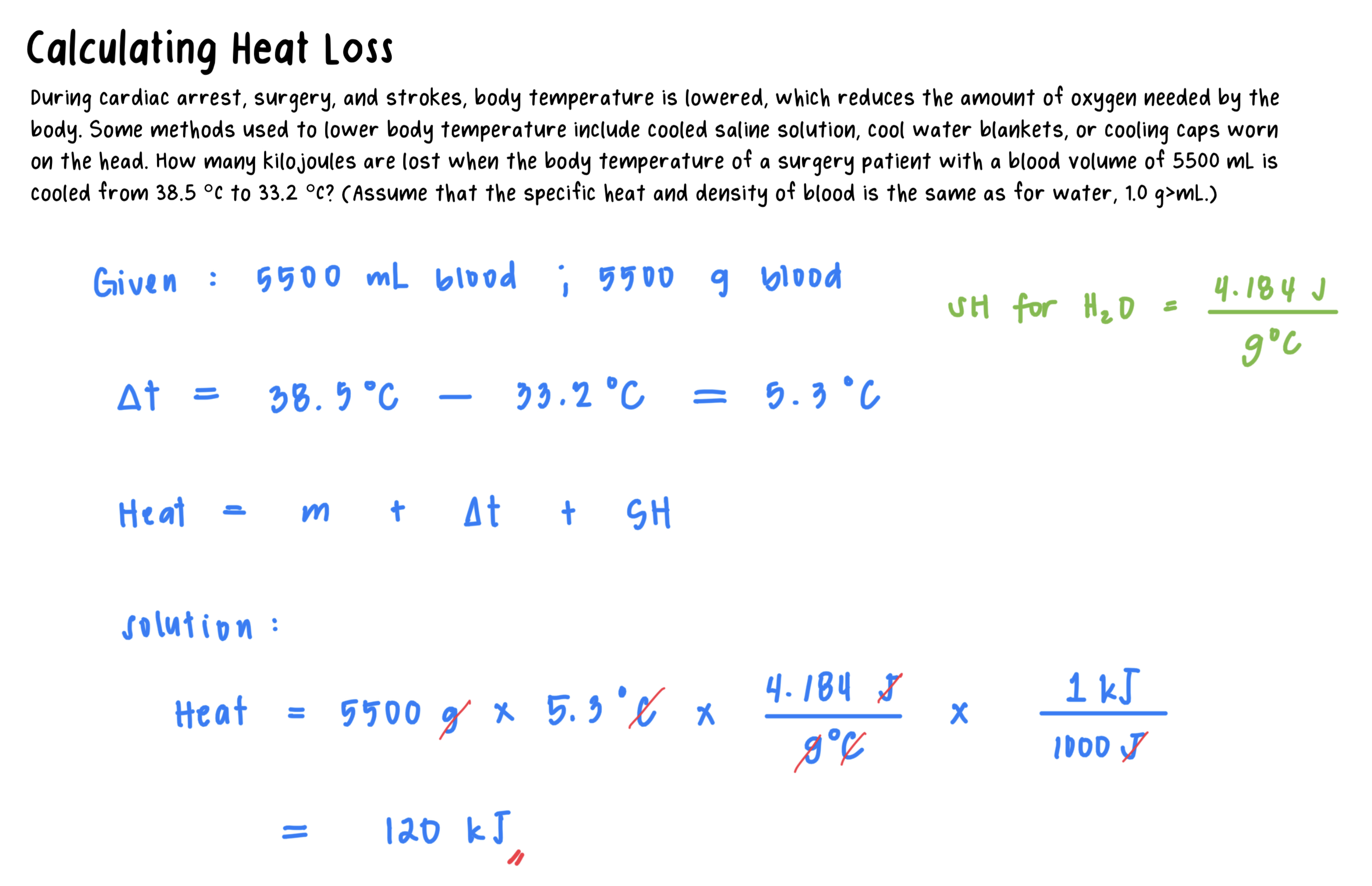
3.7: Changes of State
Change of State: It occurs when the matter is converted from one state to another.
Melting Point: The particles of a solid gain sufficient energy to overcome the attractive forces that hold them together.
The particles in the solid separate and move about in random patterns.
The substance is melting, changing from a solid to a liquid.
Freezing Point: This occurs when a liquid changes to a solid.
If the temperature of a liquid is lowered, the reverse process takes place.
Kinetic energy is lost, the particles slow down, and attractive forces pull the particles close together; therefore, the substance is freezing.
Heat Fusion: The energy that must be added to convert exactly 1 g of solid to liquid at the melting point.
The heat of fusion is also the quantity of heat that must be removed to freeze exactly 1 g of water at its freezing point.
Evaporation: It is taking place as water molecules with sufficient energy escape from the liquid surface and enter the gas phase.
Boiling Point (bp): The molecules within a liquid have enough energy to overcome their attractive forces and become gas.
Condensation: The water vapor is converted back to liquid as the water molecules lose kinetic energy and slow down.
Sublimation: The particles on the surface of a solid change directly to a gas with no temperature change and without going through the liquid state.
Deposition: The reverse of sublimation; the gas particles change directly to a solid.
Freezer Burn: This occurs when a solid is left in the freezer for a long time, and so much water sublimes that solids become dry and shrunken.
Heat of Vaporization: The energy that must be added to convert exactly 1 g of liquid to gas at its boiling point.
On a heating curve or cooling curve, the temperature is shown on the vertical axis and the loss or gain of heat is shown on the horizontal axis.
Cooling Curve: A diagram of the cooling process in which the temperature decreases as heat is removed.

Chapter 3: Matter and Energy
3.1: Classification of Matter
Matter: Anything that has mass and occupies space.
Pure Substances: A matter that has a fixed or definite composition.
Element: The simplest type of pure substance. It is composed of only one type of material.
Atoms: These are extremely tiny particles that make up each type of matter.
Compound: A pure substance that consists of atoms of two or more elements, always chemically combined in the same proportion.
Bonds: Happens when atoms are held together by attractions.
Molecules: Small groups of atoms.
Pure substances that are compounds can be broken down by chemical processes into their elements. They cannot be broken down through physical methods.
In a mixture, two or more different substances are physically mixed, but not chemically combined.
Physical processes can be used to separate mixtures because there are no chemical interactions between the components.
Homogenous Mixture: Also called a solution, the composition is uniform throughout the sample.
Heterogeneous Mixture: The components do not have a uniform composition throughout the sample.
Filtration: It helps in separating solids from liquids, which involves pouring a mixture through a filter paper set in a funnel.
Chromatography: Different components of a liquid mixture separate as they move at different rates up the surface of a piece of chromatography paper.
3.2: States and Properties of Matter
State of Matter: The physical forms of matter.
Solid: It has a definite shape and volume.
Strong attractive forces hold the particles such as atoms or molecules close together.
The particles here are arranged in such a rigid pattern, their only movement is to vibrate slowly in fixed positions.
Liquid: It has a definite volume, but not a definite shape.
The particles here move in random directions but are sufficiently attracted to each other to maintain a definite volume, although not a rigid structure.
Gas: It does not have a definite shape or volume.
The particles here are far apart, have little attraction to each other, and move at high speeds, taking the shape and volume of their container.
Physical properties
These are those characteristics that can be observed or measured without affecting the identity of a substance.
When matter undergoes a physical change, its state, size, or appearance will change, but its composition remains the same.
Examples:
Water boils to form water vapor.
Sugar dissolves in water to form a solution.
Copper is drawn into thin copper wires.
Paper is cut into tiny pieces of confetti.
Pepper is ground into flakes.
Chemical properties
These are those that describe the ability of a substance to change into a new substance.
When a chemical change takes place, the original substance is converted into one or more new substances, which have different physical and chemical properties.
Examples:
Shiny, silver metal reacts in air to give a black, grainy coating.
A piece of wood burns with a bright flame, and produces heat, ashes, carbon dioxide, and water vapor.
Heating white, granular sugar forms a smooth, caramel-colored substance.
Iron, which is gray and shiny, combines with oxygen to form orange-red rust.
3.3: Temperature
Temperatures in science, are measured and reported in Celsius (°C) units.
The freezing point of water, is defined as 0 °C, and the boiling point is 100 °C.
In the United States, everyday temperatures are commonly reported in Fahrenheit (°F) units.
Water freezes at exactly 32 °F and boils at 212 °F.
A typical room temperature of 22 °C would be the same as 72 °F.
Normal human body temperature is 37.0 °C, which is the same temperature as 98.6 °C.
Degrees: Smaller units of temperature.
Scientists have learned that the coldest temperature possible is 273 °C.
On the Kelvin scale, 273 °C temperature, which is called absolute zero, and has the value of 0 K.
Kelvins (K): The unit of the Kelvin Scale, no degree symbol is used.
3.4: Energy
Energy: The ability to do work.
Kinetic Energy: The energy of motion.
Potential Energy: Determined by the position of an object or by the chemical composition of a substance.
Heat: The energy associated with the motion of particles.
Joule (J): The SI unit of energy and work.
It is a small amount of energy and scientists often use the kilojoule (kJ).
Calorie (cal): Defined as the amount of energy needed to raise the temperature of 1 g of water by 1 °C.
3.5: Energy and Nutrition
Carbohydrates are the primary fuel for the body.
If the carbohydrate reserves are exhausted, fats and then proteins are used for energy.
In the nutrition laboratory, foods are burned in a calorimeter to determine their energy value.
The energy values for food are the kilocalories or kilojoules obtained from burning 1 g of carbohydrate, fat, or protein.
3.6: Specific Heat
Specific Heat
The amount of heat needed to raise the temperature of exactly 1 g of a substance by exactly 1 °C.
This temperature change is written as ∆T (delta T), where the delta symbol means “change in.”
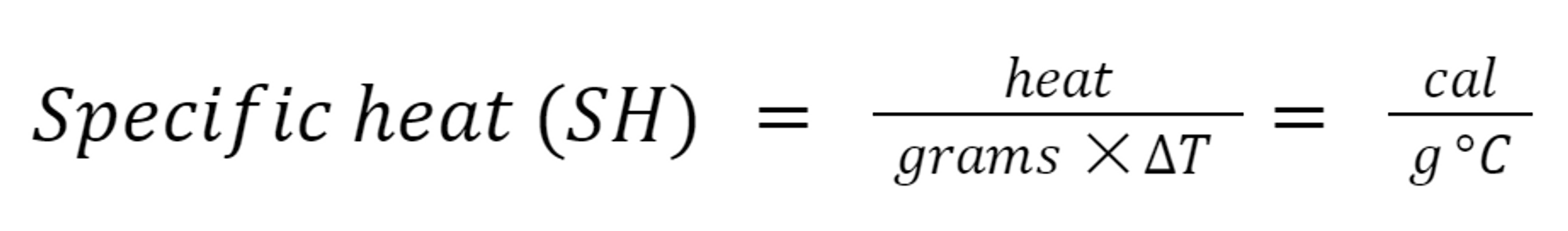
The high specific heat of the water has a major impact on the temperatures in a coastal city compared to an inland city.
A large mass of water near a coastal city can absorb or release five times the energy absorbed or released by the same mass of rock near an inland city.
Heat Equation: Specific heat expression that is arranged to solve for heat.
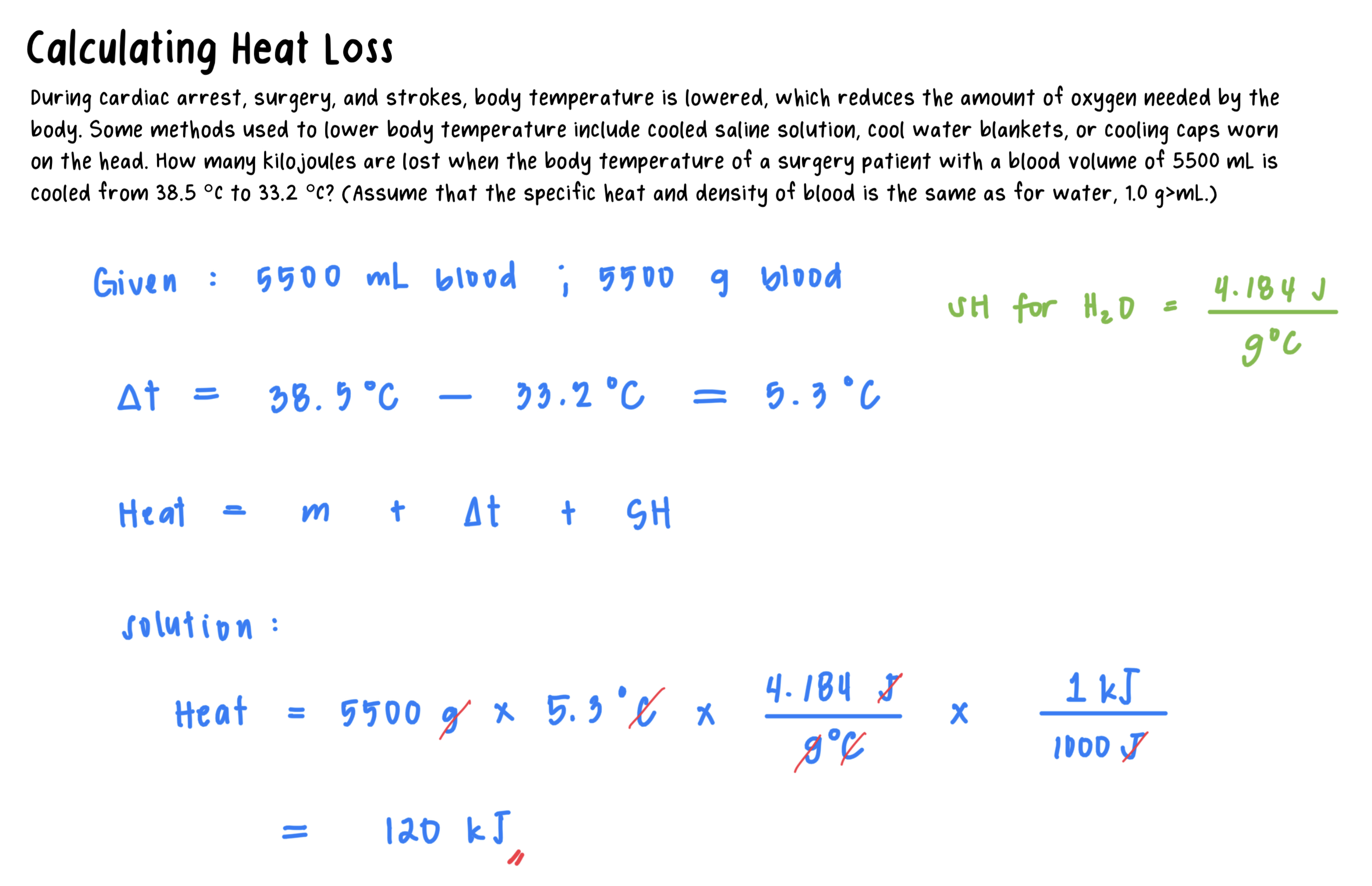
3.7: Changes of State
Change of State: It occurs when the matter is converted from one state to another.
Melting Point: The particles of a solid gain sufficient energy to overcome the attractive forces that hold them together.
The particles in the solid separate and move about in random patterns.
The substance is melting, changing from a solid to a liquid.
Freezing Point: This occurs when a liquid changes to a solid.
If the temperature of a liquid is lowered, the reverse process takes place.
Kinetic energy is lost, the particles slow down, and attractive forces pull the particles close together; therefore, the substance is freezing.
Heat Fusion: The energy that must be added to convert exactly 1 g of solid to liquid at the melting point.
The heat of fusion is also the quantity of heat that must be removed to freeze exactly 1 g of water at its freezing point.
Evaporation: It is taking place as water molecules with sufficient energy escape from the liquid surface and enter the gas phase.
Boiling Point (bp): The molecules within a liquid have enough energy to overcome their attractive forces and become gas.
Condensation: The water vapor is converted back to liquid as the water molecules lose kinetic energy and slow down.
Sublimation: The particles on the surface of a solid change directly to a gas with no temperature change and without going through the liquid state.
Deposition: The reverse of sublimation; the gas particles change directly to a solid.
Freezer Burn: This occurs when a solid is left in the freezer for a long time, and so much water sublimes that solids become dry and shrunken.
Heat of Vaporization: The energy that must be added to convert exactly 1 g of liquid to gas at its boiling point.
On a heating curve or cooling curve, the temperature is shown on the vertical axis and the loss or gain of heat is shown on the horizontal axis.
Cooling Curve: A diagram of the cooling process in which the temperature decreases as heat is removed.

 Knowt
Knowt
